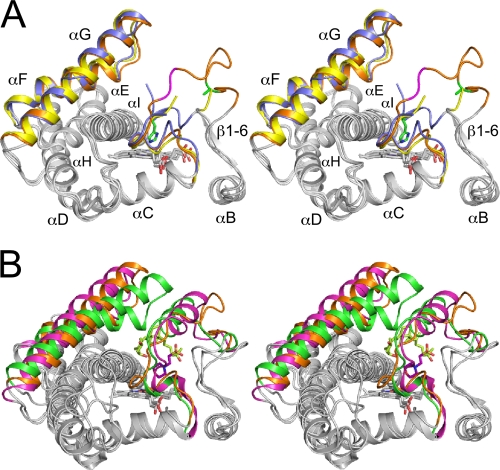
FIG. 3.
(A) Stereographic superimposition of the three crystal structures of CYP105P1. A region from B helix to I helix is shown as ribbon models. FG helices and BC loop regions of WT-free, WT-4PI, and H72A-free structures are colored in blue, yellow, and orange, respectively. Two residues in the BC loop of H72A-free structure (Gly80 and Lys81 colored in magenta) exhibit relatively weak electron density. The side chains of His72 in WT-free and Ala72 in H72A-free structures are shown as stick models with carbon atoms green. (B) Comparison of H72A-free structure with two CYP105 structures, P450 SU-1 in complex with 1α,25-dihydroxyvitamin D3 (PDB code 2ZBZ) and P450 MoxA in complex with MES (PDB code 2Z36). FG helices, BC loop regions and bound ligands of CYP105P1, P450 SU-1, and P450 MoxA are colored orange, green, and magenta, respectively.