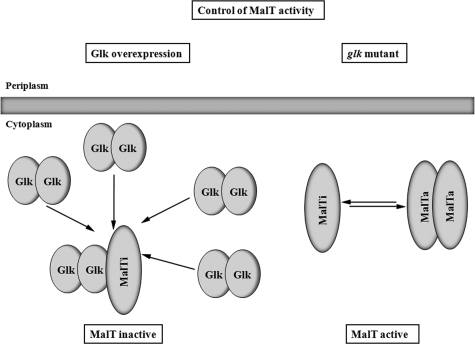
FIG. 7.
Binding of Glk to MalT inhibits MalT activity. Two situations are shown. On the right-hand side, the “normal” situation is shown. MalT exists in an equilibrium of the inactive monomer and the active dimer (multimer). On the left-hand side, the overproduction of glucokinase is shown. In the proposed model, high concentrations of Glk result in the binding of Glk dimers to monomeric and inactive MalT, preventing mal gene expression. Not shown in this scheme is the essential role of MalP or MalQ in Glk-dependent MalT inhibition. Also not shown is the role of maltotriose in preventing the inhibition by Glk. The established inhibition of MalT by other proteins such as Aes, MalK, or MalY is not shown but was the basis for this model. Also novel is the proposal that even in the absence of the inducer maltotriose MalT can form a transcriptionally active species.