Summary
Antifreeze proteins (AFPs) lower the noncolligative freezing point of water in the presence of ice below the ice melting point. The temperature difference between the melting point and the noncolligative freezing point is termed thermal hysteresis (TH). The magnitude of the TH depends on the specific activity and the concentration of AFP, and the concentration of enhancers in the solution. Known enhancers are certain low molecular mass molecules and proteins. Here, we investigated a series of polycarboxylates that enhance the TH activity of an AFP from the beetle Dendroides canadensis (DAFP) using differential scanning calorimetry (DSC). Triethylenetetramine-N,N,N′,N″,N‴,N‴-hexaacetate, the most efficient enhancer identified in this work, can increase the TH of DAFP by nearly 1.5 fold over than that of the published best enhancer, citrate. The Zn2+ coordinated carboxylate results in loss of the enhancement ability of the carboxylate on antifreeze activity. There is not an additional increase in TH when a weaker enhancer is added to a stronger enhancer solution. These observations suggest that the more carboxylate groups per enhancer molecule the better the efficiency of the enhancer and that the freedom of motion of these molecules is necessary for them to serve as enhancers for AFP. The hydroxyl groups in the enhancer molecules can also positively affect their TH enhancement efficiency, though not as strongly as carboxylate groups. Mechanisms are discussed.
Keywords: Antifreeze protein, Thermal hysteresis activity, Antifreeze protein activation, Differential scanning calorimetry
1. Introduction
Antifreeze proteins are found in polar fishes [1], insects [2, 3], centipedes [4], mites [5], spiders [6], fungi, bacteria [7] and many plants [8]. AFPs exhibit remarkable structural variation among species. Three types of insect AFPs have been structurally characterized so far. They have repeat structures and often occur as a series of isoforms without close similarities to any other known protein. The moth AFP from the spruce budworm Choristoneura fumiferana (CfAFP) is a threonine- and serine-rich left-handed parallel β-helical protein that has 15 residues per coil [9, 10]. The AFPs from the fire-colored beetle Dendroides canadensis (DAFP) and the mealworm Tenebrio molitor (TmAFP) are another type of insect AFP. They are extensively disulfide bonded right-handed β-helical proteins [11, 12]. The cleavage of the disulfide bonds results in the loss of antifreeze activity. The two beetle AFPs have the same number of Cys residues at the same positions and have 48–67 % identity at the amino acid level [13]. CfAFP, TmAFP, and DAFP all contain a Thr-X-Thr motif. Most recently, a third type of insect AFP which is glycine-rich, was identified in a snow flea. This AFP lacks structural similarity and produces a different ice crystal morphology than those of any reported AFPs in fish, plants, or insects [14, 15].
Though the structures of AFPs from the different species often differ greatly, they all depress the non equilibrium freezing points of water without appreciably changing the melting point of ice. This temperature difference between the melting point and the freezing point is termed thermal hysteresis (TH) [16]. The generally accepted mechanism of AFPs is the adsorption-inhibition mechanism [17], that is AFPs adsorb onto the ice crystal surface at preferred growth sites [17]. The details of the means by which AFPs bind to ice are somewhat controversial, and probably depend to some extent on the type of AFP. Proposed mechanisms involve hydrophobic and van der Waals interactions [18–20] and/or hydrogen bonding [21–23]. The adsorption of AFPs on the ice surface only allows ice crystal growth to take place in highly curved fronts, whose surface free energy is higher than that of the usual low radius of curvature fronts in the absence of AFPs. The ice crystal growth process is thereby halted by the Kelvin effect [17].
During the winter, larvae of the beetle Dendroides canadensis produce antifreeze proteins (DAFPs) that prevent freezing in these freeze avoiding insects by inhibiting inoculative freezing across the cuticle by external ice and by inhibiting ice nucleators in the hemolymph and gut [24]. Several isoforms of these DAFPs have been found [13, 25]. DAFPs consist of 12 or 13 mer repeats with a size of 7.3–16.2 kDa. Every sixth residue is a cysteine, which forms disulfide bridges [26]. Although the specific activities of DAFPs are greater than those of most other AFPs, it is suggested that the mechanism of action is also adsorption-inhibition [26]. A molecular model of DAFP suggested that the ice-binding β sheet of DAFP, similarly to that of TmAFP, is made up of aligned Thr-Cys-Thr motifs matching the oxygen atoms on the prism face of ice crystal [20]. According to the molecular model, the hydroxyl groups of the Thr occupy the positions of the oxygen atoms on the neighboring ice crystal surface without any steric conflicts while forming at least three hydrogen bonds to the oxygen atoms in the ice lattice. These results suggest that the mechanism of DAFP antifreeze activity may be an adsorption-inhibition process through hydrogen bonds.
The TH values of AFPs depend on the specific activity of the AFP and its concentration until the maximum activity plateau is reached. Usually the DAFPs have relatively high TH values of 5–6 °C in the hemolymph, but some individuals have TH values of 8–9 °C [27]. However, the highly purified individual DAFPs were found to have much lower TH values, even at very high protein concentrations [28]. The TH activities of DAFPs can be strongly affected by the presence of other substances. The TH values can be enhanced by solutes of low molecular mass [26], antibodies [29], or protein enhancers [25, 30]. The protein or antibody enhancers may increase the TH activities of DAFPs by interacting with the DAFPs that bind to the surface of the ice crystal. Therefore, the surface area of the ice crystal blocked by the DAFPs is increased [25, 29]. The low molecular weight enhancers may optimize the higher-order structure of the AFPs for binding to ice by providing subtle stabilization [12]. In addition, the low molecular weight enhancer, glycerol, may further the enhancement by promoting interactions between DAFPs and other protein enhancers [25, 30]. Here, we describe an investigation of the TH activities of purified DAFP-1 in the presence of some polycarboxylates as potential low molecular weight TH enhancers using differential scanning carlorimetry (DSC). For the first time, we correlate the efficiency of the TH enhancer molecules with their structural and physiochemical properties and demonstrate that the number of carboxylate and/or hydroxyl groups in the enhancer molecules affects their TH activity enhancement ability for beetle AFPs. Possible explanations are discussed.
2. Materials and methods
2.1 Materials
The sodium salts of polycarboxylic acid (PCA) and aminopolycarboxylic acid (PACA) were purchased from Sigma-Aldrich Chemical Co. and used without additional purification. All the solutions were prepared using Milli-Q water produced from a Synergy water system (Millipore Co.) with a minimum resistivity of 18 MΩ·cm. The samples were in 50 mM sodium phosphate buffer, pH 7.40 unless otherwise indicated. The samples were filtered through a 0.1 μm filter before use.
2.2 DAFP-1 sample preparation
The E. coli Origami B cells harboring pET32b-DAFP-1 were grown in Luria-Bertani media supplemented with 15 μg/mL kanamycin and 50 μg/mL ampicillin. When the absorbance at 600 nm reached 0.6, isopropyl-1-thio-β-D-galactopyranoside (IPTG) was added into the culture to a final concentration of 0.5 mM in order to induce a high level expression of DAFP-1. Then cells were harvested by centrifugation at 4 °C after a 30 hour incubation at 15 °C. The cells were disrupted with a French press (Thermo Fisher) at 1250 psi two to three times. The crude protein was purified using Nickel affinity resin. DAFP-1 was concentrated and subjected to a final purification step by AKTA Purifier 10 (GE Healthcare) using a Sephacryl S-100 gel filtration column (GE Healthcare). The above procedure was modified from a literature procedure [25].
2.3 TH activity measurements
A DSC 823e (Mettler Toledo, OH) with an HSS7 high sensitivity sensor and a Julabo FT900 intracooler chiller (Julabo Company, PA) was used in this study. The sensitivity of the DSC 823e device is sub μW. Empty aluminum pans were used for baseline calibration. A two point temperature calibration was performed using gallium and octane before all the TH measurements. Since the DSC experiments were conducted over a broad temperature range, the pH of the buffer should have little temperature dependence in an ideal case. Therefore, sodium phosphate (NaPi) buffer (50 mM, pH 7.40) is used to minimize the temperature dependence on the pH of the experimental samples [31].
Although microscope techniques that permit direct observation of the melting and growth of ice crystals have most often been used to measure TH values, differential scanning calorimetry (DSC) has been extensively used to study ice formation in biological samples, and DSC also provides an accurate method to analyze the TH activity of AFPs [32–37]. By measuring the difference in the energy necessary to establish a nearly zero temperature difference between a sample and an inert reference, the exothermic and endothermic events during state changes of the sample can be monitored.
About 3.5 μL of sample was placed in an aluminium pan. The weight of the sample was recorded using an analytical balance. An empty aluminum pan was placed in the reference cell. The TH activity of DAFP-1 was characterized by DSC as described in detail elsewhere [33, 35, 38]. Briefly, a sample was cooled to −40 °C (completely frozen), held at −40 °C for 5 minutes to allow the system to stabilize, and then slowly heated at a rate of 1.00 °C min−1 until completely melted. The melting point and the melt endothermic area (the enthalpy of melting) were recorded. The sample was then cooled to −40 °C again, held at −40 °C for 5 minutes, and then slowly heated at a scan rate of 1.00 °C ·min−1 to partially melt the sample. After holding at this temperature (Th) for 5 minutes, the sample was then slowly cooled at the rate of 1.00 °C ·min−1 to completely refreeze. The onset temperature (To) of the crystallization and the refreeze exothermic area (the enthalpy of ice-inoculated freezing) was recorded.
The percentage of the ice (% ice) in the sample was estimated as [1−(− ΔHf/ΔHm)] × 100 %, where ΔHm is the enthalpy of melting and −ΔHf is the enthalpy of ice-inoculated freezing [33]. Scans at different Th may result in different ice fractions in the sample, which may affect the measured TH activities [36, 39]. The difference between the Th and the To is usually represented as the TH activity determined using the DSC method to quantitatively assess the TH activities at various ice fractions [32, 33, 36]. In this work, the TH activities (apparent TH) were calculated as the difference between the Th and the To for all the samples at very small ice fractions (~7 %). The experiment at each Th was repeated at least twice. The TH values were presented as means and the standard deviations were between 0.01 and 0.02 °C.
2.4 TH activity of DAFP-1 in the presence of enhancers
The sodium salts of the polycarboxylic acids, NaAc, HBA, GTA, TCA, CIT, Iso-CIT, BTCA and the sodium salts of the aminopolycarboxylic acids, NTA, EDTA, EGTA, DTPA, TTHA were tested as potential enhancers of DAFP antifreeze activity. Sodium chloride was used as a control. We measured the TH activities of DAFP-1 in the presence of the the above compounds using the DSC method described in section 2.3. The concentration of DAFP-1 in all the samples was 0.669 mM. The tested concentrations of the compounds were from 0.00 to 0.80 or 1.00 M. (Non-physiologically high concentrations of carboxylic acid salts and aminocarboxylic acid salts were used in these studies.) The TH activity of DAFP-1 was also measured in the presence of a combination of two compounds. Three groups of combinations were tested: (A) GTA (as a stronger enhancer in the system) and NaAc (as a weaker enhancer); (B) GTA (as a stronger enhancer in the system) and HBA (as a weaker enhancer); (3) EDTA (as a stronger enhancer in the system) and CIT (as a weaker enhancer). The concentrations of the individual compounds used in the combinations varied between 0.00–0.80 or 1.00 M.
3. Results
3.1 Characterization of the TH activity of DAFP-1
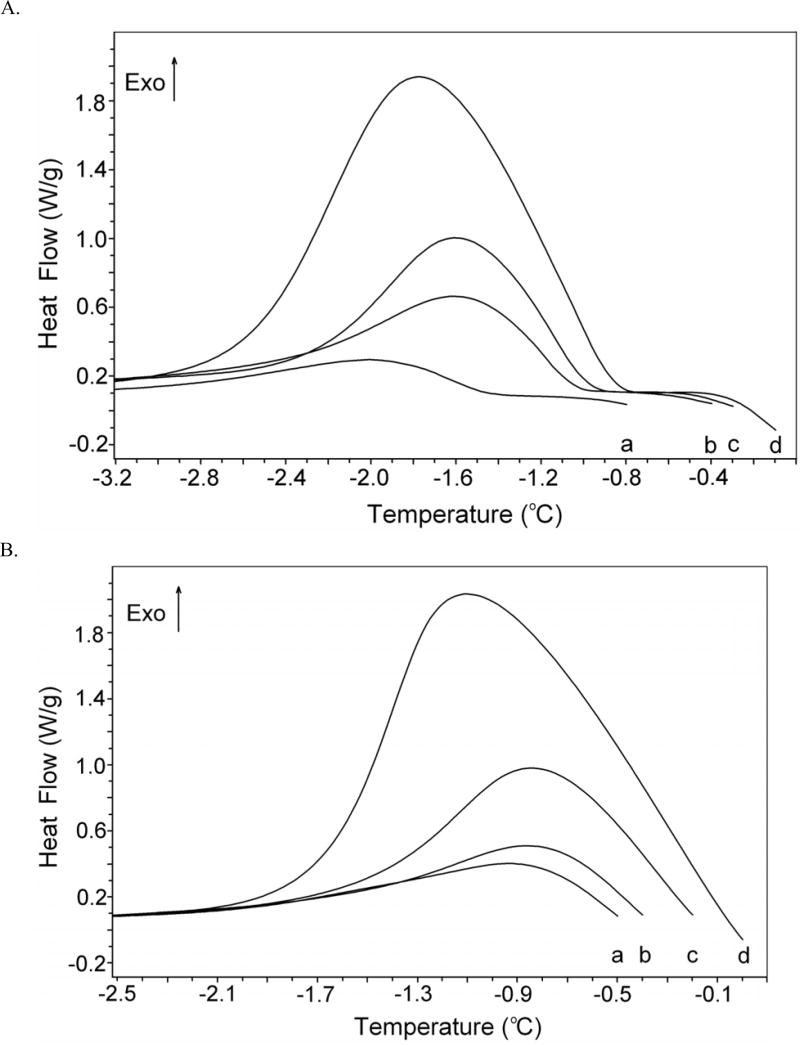
The scan procedure was repeated at different hold temperatures (Th) for DAFP-1 and BSA samples and the resulting ice fractions in the samples were from 24 % to 7 % (Fig. 1). TH activity was observed in the DAFP-1 sample at these ice fractions. For example, curve d in Fig. 1A shows a DSC scan at 7 % ice, where Th was −0.10 °C and To was −0.75 °C. Thus the TH of the DAFP-1 sample was 0.65 °C (apparent). As reported previously, the TH activity of the DAFP-1 was decreased when the ice fraction was increased (curves a–d, Fig. 1A) [32, 33, 35, 36]. By holding the temperature (Th) 0.3 °C lower than the melting point of a sample, the % ice in the sample was kept to a minimum (~7 % in this study) in order to minimize the effect of % ice on the TH activity. Thus the difference between the TH reported here (apparent) and the theoretical TH values were 0.3 °C for all the samples. As a control, no TH activity was observed in the BSA sample (Fig. 1B). The sodium phosphate buffer (NaPi) alone does not have TH activity. The effect of pH on the activity of DAFP-1 was also determined. The TH activity of DAFP-1 was unchanged from pH 4.0 to pH 10.0 (data not shown), which is consistent with the published data [12]. The results suggest that different ionization states of charged side chains of DAFP-1 may not affect the TH values of a measured solution.
Fig. 1.
Differential scanning calorimetry (DSC) thermograms. (A) DSC thermograms of partially melted DAFP-1 (0.669 mM) at a rate of 1.00 °C/min illustrating its TH activity. The hold temperatures (Th) are indicated as positions a–d in the graph, respectively, are −0.80, −0.40, −0.30, and −0.10 °C. (B) DSC thermograms of partially melted BSA (0.303 mM) at a rate of 1.00 °C/min. The hold temperatures (Th) indicated as positions a–d in the graph, respectively, are −0.50, −0.40, −0.20, and 0.00 °C. The estimated percentages of the ice in the two samples are between 24 % and 7 %.
3.2. Effects of polycarboxylates and aminopolycaboxylates on DAFP-1 activity
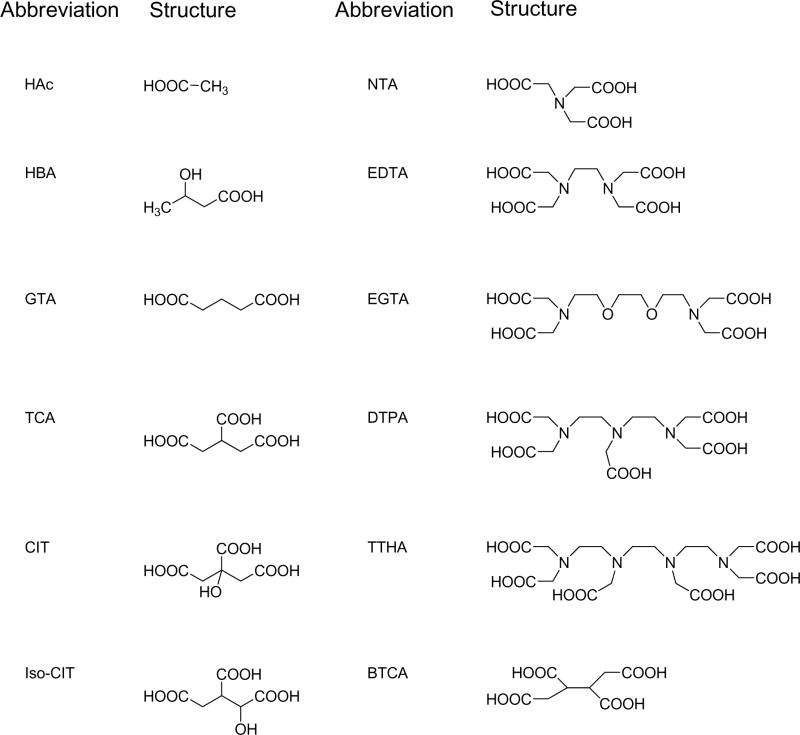
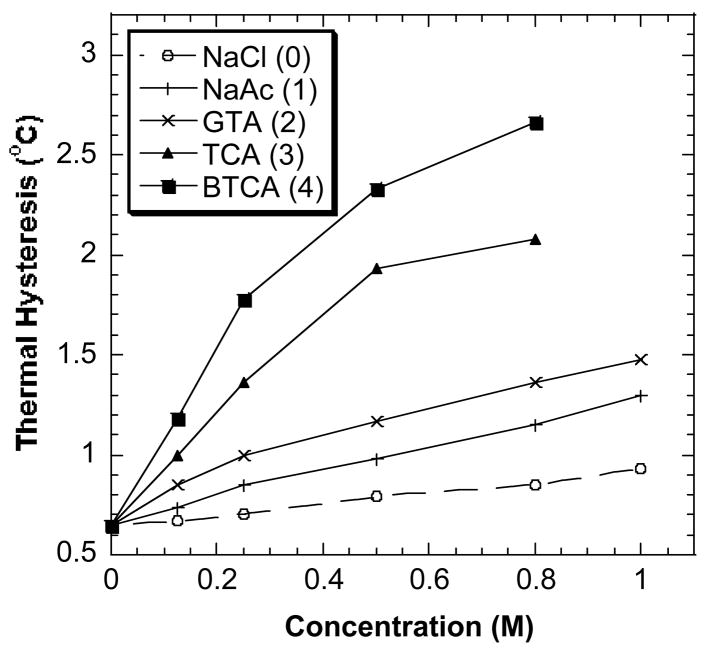
The enhancement effects of a series of polycarboxylates and aminopolycarboxylates on the TH activity of DAFP-1 were tested. The structures of the selected polycarboxylates and aminopolycarboxylates are listed in Fig. 2. It has been reported that CIT was the most efficient low molecular weight enhancer of TH activity for DAFPs identified so far [12]. Here, we show that some polycarboxylates and the aminopolycarboxylates containing more carboxylate groups are more active TH activity enhancers than CIT. Compared to that in the presence of NaCl, the TH activity of DAFP-1 is increased in the presence of the selected polycarboxylates (Fig. 3). In addition, the enhancing ability of the compounds is BTCA (4) > TCA (3) > GTA (2) > NaAc (1). The number indicated in the parentheses after the compound is the number of the carboxylate groups present in the compound. These results suggest that the greater the number of carboxylate groups per molecule the better the efficiency of the enhancer.
Fig. 2.
The abbreviations and the acid structures of the selected compounds. The sodium salts of selected polycarboxylic acids and aminopolycarboxylic acids were used as potential TH enhancers for the antifreeze protein from Dendroides canadensis, DAFP-1.
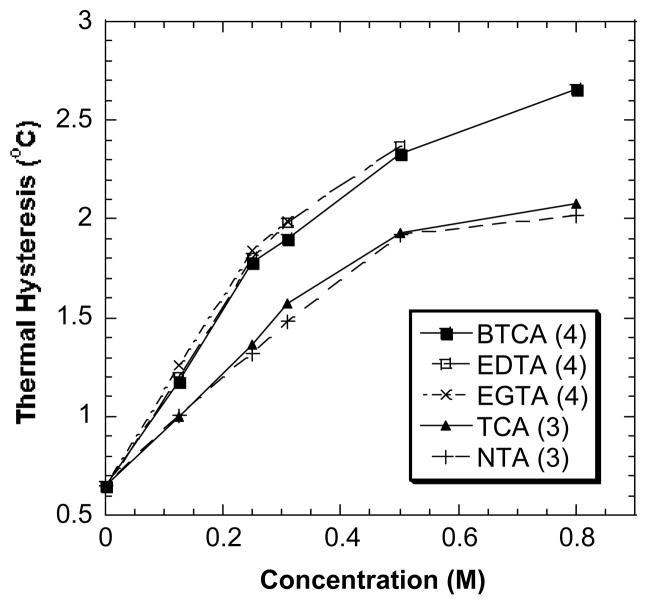
Fig. 3.
The TH activity of DAFP-1 in the presence of the sodium salts of selected polycarboxylic acids. Sodium chloride was plotted as a control. All the samples contained 0.669 mM DAFP-1 in 50 mM sodium phosphate buffer, pH 7.40. The concentrations of the compounds were tested from 0.00 to 0.80 or 1.00 M. The numbers in parentheses are the numbers of carboxylate groups in the compounds.
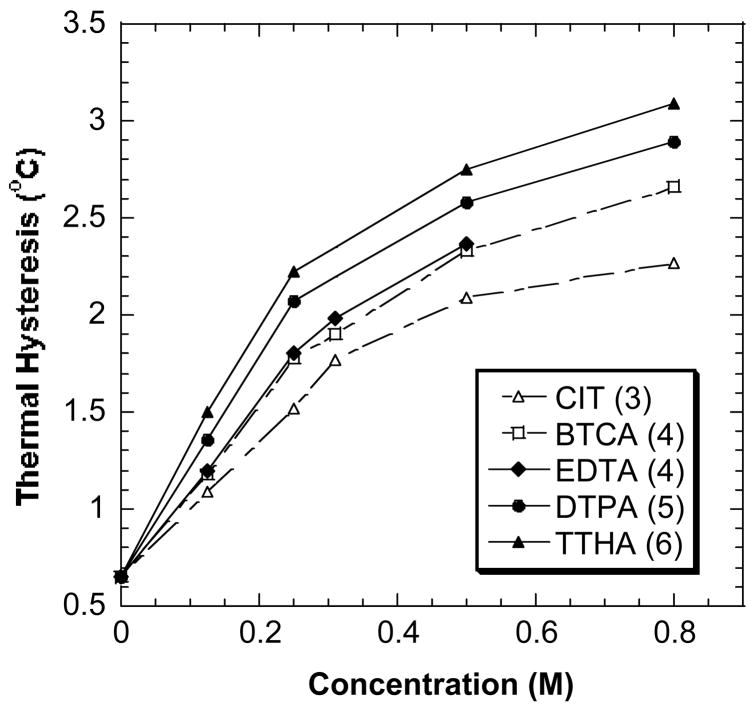
The above trend has been observed in all our experiments. As shown in Fig. 4, the order of the enhancing ability of the tested compounds is TTHA (6) > DTPA (5) > EDTA (4) ~ BTCA (4) > CIT (3). (The solubility of EDTA at pH 7.40 limited the TH activity measurement of EDTA at higher concentrations.) TTHA containing six carboxylate groups shows ~1.5 fold higher enhancing ability than CIT, which was reported as the best low molecular weight enhancer for DAFPs [12]. While the number of carboxylate groups per individual molecule may be one of the main factors that contribute to the enhancement abilities of low molecular weight enhancers in TH activity, the fold increase in TH activity enhancement by the enhancers seems not to relate directly to the fold increase in the number of carboxylate groups in the enhancers. For example, the ratio of the number of the carboxylate groups in TTHA and BTCA is 3 to 2, but the enhancement ratio is about 4 to 3.
Fig. 4.
The TH activity of DAFP-1 in the presence of the sodium salts of polycarboxylic acid and aminopolycaboxylic acids. The compounds plotted in this graphs were identified as better TH activity enhancers than CIT (the published best enhancer). The data of CIT were also plotted for references. The concentrations of the compounds were tested from 0.00 to 0.80 M. The numbers in parentheses are the numbers of carboxylate groups in the compounds.
Fig. 5 shows that BTCA, EDTA and EGTA have very similar enhancement effects on the TH activity of DAFP-1 at the same concentrations. BTCA, EDTA and EGTA all have four carboxylate groups. Because of the limited solubility of EDTA and EGTA at pH 7.40, we can only study its enhancing effects under relatively lower concentrations. Similarly, TCA and NTA salts have nearly equal effects as enhancers for DAFP-1 at the same concentrations and both TCA and NTA contain three carboxylate groups. These results again suggest that carboxylate groups play a key role in the DAFP-enhancing mechanism of these polycarboxylates and aminopolycarboxylates, while the nitrogen or oxygen atoms inside carbon chain may not affect the enhancing ability of the enhancers appreciably.
Fig. 5.
The effect of carboxylate groups and heteroatoms in the carbon chains in the polycarboxylate and aminopolycarboxylate TH enhancers on their TH enhancement ability. The numbers in the parentheses are the numbers of the carboxylate groups in the compounds.
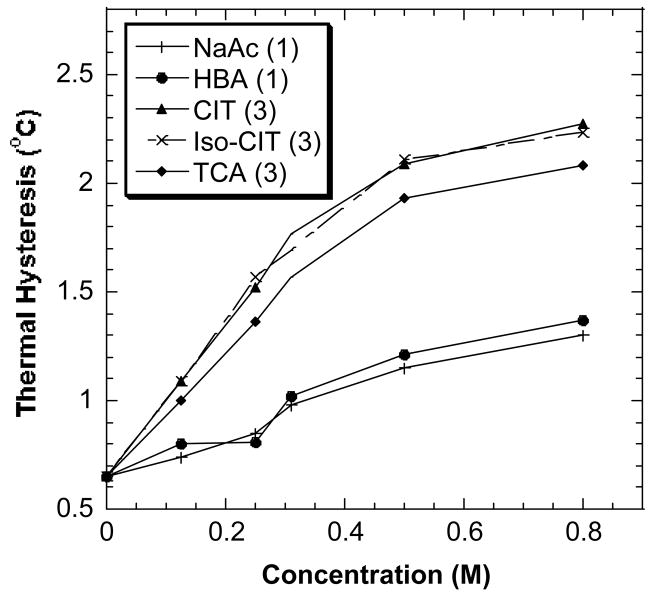
Fig. 6 also demonstrates that different carboxylates have very similar enhancement abilities on the TH activity of DAFP-1 if their acids contain the same number of the carboxylate groups. Both CIT and Iso-CIT have the same number of carboxylate groups (three), and their salts have very similar AFP enhancing ability. Similarly, NaAc and HBC have one carboxylate group, and their salts have very similar enhancing ability. However, TCA also has three carboxylate groups like CIT and Iso-CIT, but exhibits a lower enhancing ability than CIT or Iso-CIT. Furthermore, HBA has the same number of carboxylate groups as HAC, but has a higher TH activity enhancing ability. Therefore, presence of hydroxyl groups in the molecules seems to play a role in the TH activity enhancement ability of these enhancers, but the position of the hydroxyl group does not. Unlike CIT (or Iso-CIT) and HBA, TCA and NaAc lack a hydroxyl group. The results indicate that hydroxyl groups do play a role in the TH activity enhancement abilities of the DAFP enhancers, but the effects of hydroxyl groups are relatively smaller than those of carboxylate groups.
Fig. 6.
The effect of carboxylate and hydroxyl groups in the polycarboxylate enhancers on their TH enhancement ability. The numbers in the parentheses are the numbers of the carboxylate groups in the compounds.
3.3. Effects of enhancer-Zn complexes on DAFP-1 activity
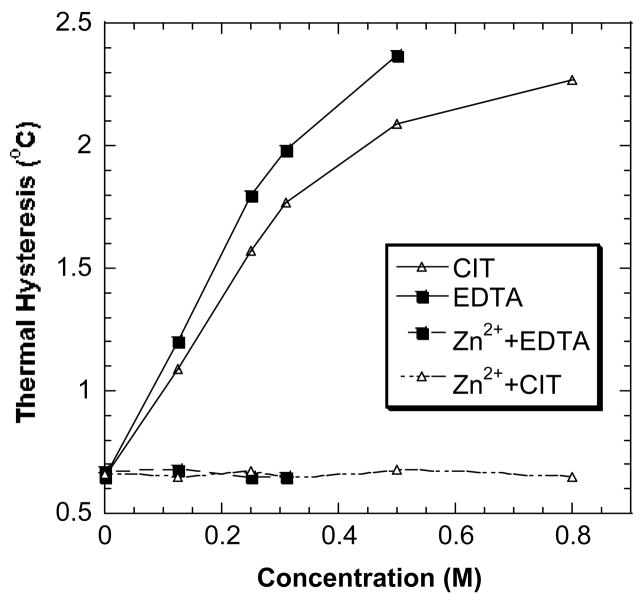
Fig. 7 demonstrates that the addition of Zn2+ diminished the enhancing capabilities of CIT and EDTA. The final molar ratios of EDTA:Zn2+ and CIT:Zn2+ were 4:1 and 6:1, respectively. The addition of Zn2+ to EDTA and to CIT caused the formation of Zn(II)-EDTA and Zn(II)-CIT complexes, which diminished the TH activity enhancement abilities of EDTA and CIT. The resulting TH values of DAFP-1 in the presence of the above solutions were nearly the same as that of DAFP-1 alone.
Fig. 7.
The effect of the addition of Zn2+ to CIT and EDTA in DAFP-1 solutions (0.669 mM). The molar ratio of EDTA to Zn2+ was 4:1 and the molar ratio of CIT to Zn2+ was 6:1. The formation of Zn(II)-EDTA and Zn(II)-CIT complexes may diminish the TH enhancement abilities of EDTA and CIT, respectively.
3.4. Effects of enhancer combinations on DAFP-1 activity
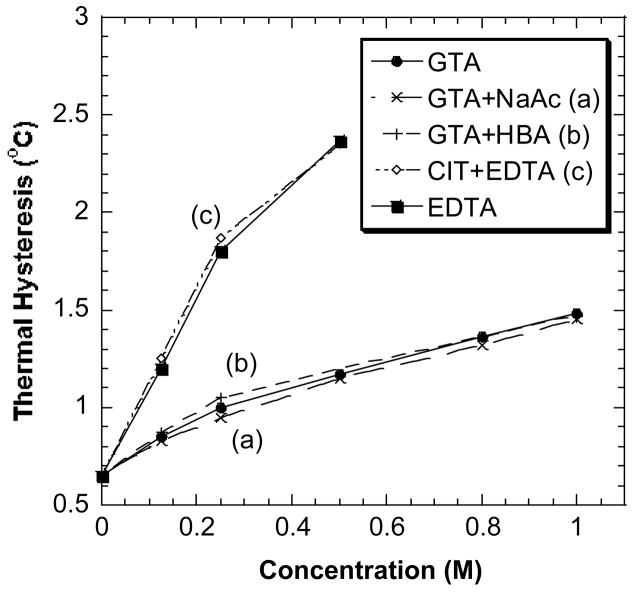
The TH activities of DAFP-1 in the presence of combinations of a stronger and a weaker enhancer are plotted with those in the presence of the single compound in Fig. 8. Compared to the effect of the stronger enhancer alone on the TH activity of DAFP-1, there is no appreciable increase in the TH values of the DAFP-1 solutions in the presence of both the stronger enhancer and a weaker enhancer: GTA + HAc ~ GTA, GTA + HAc ~ GTA, and EDTA + CIT ~ EDTA (Fig. 8). For example, the TH activity of DAFP-1 in the presence of 0.50 M EDTA and 0.50 M CIT is 2.35 °C, which is nearly the same as that in the presence of 0.50 M EDTA alone (TH = 2.37 °C) (Fig. 8). Similarly, the TH activity of DAFP-1 in the presence of 0.50 M GTA and 0.50 M HBA is 1.20 °C, which is nearly the same as that in the presence of 0.50 M GTA alone (TH = 1.17 °C). Other combinations of weak and strong enhancers had similar effects (data not shown).
Fig. 8.
The effect of combinations of a weaker enhancer and a stronger enhancer on the TH activity of DAFP-1. (a) GTA (as a stronger enhancer in the system) and NaAc (as a weaker enhancer); (b) GTA (as a stronger enhancer in the system) and HBA (as a weaker enhancer); (c) EDTA (as a stronger enhancer in the system) and CIT (as a weaker enhancer). The concentrations of the individual enhancers in the combinations were from 0.00 to 0.80 or 1.00 M. The data of GTA and EDTA were plotted as references in the graph.
The polycarboxylic acid salts and the aminopolyarboxylic acid salts that were used in this study lack TH activity on their own, but they do, of course, have the normal colligative effect of depressing the melting/freezing point. The results with the combinations of strong and weak enhancers with DAFP-1 indicated that a weaker enhancer plays an insignificant role in the presence of a stronger enhancer. Interestingly, the ionic strengths of the DAFP-1 solutions in the presence of the combinations are larger than those in the presence of the single enhancer, but the TH values of the two cases are very similar. This suggests that the ionic strength of the solution may not significantly affect the TH activity of DAFP-1.
4. Discussion
The presence of other solutes, including other proteins, can enhance the TH activities of DAFPs. These enhancers include low molecular mass solutes [12], other proteins [30], and even other DAFPs [25]. Consequently, in the hemolymph of winter D. canadensis larvae the mean TH is generally 3–5°C, due to the presence of DAFPs-1 –2, -4, and –6 which interact with one another, resulting in self-enhancement [25]. In addition, the presence of thaumatin-like protein and high concentrations of glycerol (0.5–1.0 M) that promote the interactions of the proteins provide further enhancement. Consequently, the TH activity of a purified single DAFP, such as DAFP-1, is typically lower than that measured in the hemolymph. The concentrations of low molecular mass enhancers required for enhancement is approximately 250–500 mM. Therefore, glycerol is the only small enhancer naturally present at sufficiently high concentrations in D. canadensis to act as an enhancer. While some of the polycarboxylates shown in this study to have TH enhancer activity (i.e., CIT and Iso-CIT) are present in biological systems, they do not occur in sufficiently high physiological concentration to function as natural enhancers of DAFP activity.
Among the low molecular mass enhancers identified in previous studies [12], citrate had the greatest enhancement activity. The study reported here was undertaken to further investigate the factors responsible for the TH enhancement of citrate and other polycarboxylates. The order of the number of carboxylate groups per single molecule is consistent with the enhancing capabilities of the molecule. Compounds with only one or two carboxylate groups are less effective than CIT (3). Compounds with 4–6 carboxylate groups such as TTHA (6), DTPA (5), EDTA (4), and BTCA (4) are more efficient enhancers. TTHA is the most efficient TH activity enhancer of DAFP-1 that was identified in this work. Usually optimal TH activity enhancement effects of AFP enhancers are obtained at relatively high concentrations [12]. Thus the solubilities of the TH activity enhancers of AFPs are also important for their application. The lower solubilities of EDTA and EGTA may limit their application.
Nitrogen or oxygen atoms inside the carbon chain of the enhancers do not affect their TH enhancement abilities. The polycarboxylates and aminopolycarboxylates containing the same number of carboxylate groups have a similar effect on the TH activity enhancement. BTCA, EDTA, and EGTA each have four carboxylate groups. BTCA does not contain any nitrogen or oxygen atoms in its carbon chain, while EDTA contains two nitrogen atoms and EGTA has two nitrogen atoms and two oxygen atoms, respectively. Their TH enhancement abilities are similar.
The hydroxyl group (-OH) also plays a role in TH enhancement activities, although the effect of the hydroxyl group is smaller than that of the carboxylate group. The results indicate that the enhancers containing more hydroxyl groups have slightly higher TH activity enhancement ability.
Zn2+ can coordinate with many carboxylic acids. For example, the formation constants (log K) for Zn-EDTA and Zn-CIT are 16.5 and 4.9, respectively [40, 41]. The addition of Zn2+ to EDTA and to CIT resulted in diminished TH activity enhancement ability. The TH activity of the DAFP-1 solution in the presence of the Zn-EDTA or the Zn-CIT complex was nearly the same as that of the DAFP-1 solution alone. When EDTA or CIT complexes with Zn2+, some or all of their carboxylate groups bind to the metal ion and restrict their freedom of motion. This may be an explanation of the diminished enhancing ability of the Zn-EDTA and the Zn-CIT complexes. These results also suggest that the freedom of motion of the enhancers plays an important role in their TH activity enhancement abilities. In order to determine whether the loss of the negative charge of the enhancer is responsible for the loss of enhancer activity, we measured the TH activity of the DAFP-1 solution alone, and in the presence of BTCA, at a few different pHs. The TH values of the solutions were nearly equal, within experimental error, at pHs 4.00, 6.00, 8.00, and 10.00. Thus the loss of enhancer activity may not due to the loss of the negative charge of the enhancer.
It should be mentioned that, compared to DAFP-1 in solution with NaCl, DAFP-1 in the presence of polycarboxylates or aminopolycarboxylates does not significantly affect the viscosities of the specific solutions. Also, the presence of the C-terminal tag in the DAFP-1 construct does not affect the activity of DAFP-1 or the activity of DAFP-1 in the presence of the enhancers. In addition, the tag alone does not have any TH activity in NaPi buffer or in the presence of the enhancers (Amornwittawat, N. and Wen, X. unpublished data).
According to the widely-accepted adsorption-inhibition mechanism for the TH activity of AFPs [17], the plateau of the TH activity is reached once AFP molecules are saturated on the ice surface and additional TH activity is not observed when more AFP molecules are added. A similar process may occur in AFP solutions in the presence of enhancers. As more enhancer molecules are added to an AFP solution, the TH activity of the solution increases until it reaches the maximum TH activity [12]. A possible explanation for this phenomenon is that enhancer molecules may result in AFP molecules adsorbing to ice crystal surfaces more efficiently, thereby increasing the coverage of the ice crystal surface, adsorbing to more ice planes, or forcing the ice crystal growth into more highly curved fronts. Any of these actions may result in the increases of TH activity [17, 20, 29]. A plateau of TH activity is thus formed when the AFP molecules with the enhancer molecules saturate the ice surface. Little additional increase in TH values was observed when a weaker enhancer was added to a stronger enhancer solution. A weaker enhancer seems to play an insignificant role in the presence of a stronger enhancer in the solution. (The result also indirectly suggests that the ionic strength of the solution may not have much effect on the TH values.) In addition, there is a possibility of direct interactions between AFP molecules and enhancer molecules. The interaction might be competitive that is the stronger enhancer may exclude the weaker enhancer. We have determined that some of the enhancers can bind to DAFP-1 (Wang, S. and Wen, X., unpublished data), leading to the possibility that the enhancers may produce aggregations of DAFPs that, because of their larger size may better block the surface of the ice crystal. Enhancement of the antifreeze activity by promoting aggregation of AFPs has been reported previously [25, 42]. For example, glycerol further enhances the antifreeze activity of DAFPs by promoting interactions among the different DAFPs present in the experimental solution [25]. The polycarboxylate enhancers might have a mechanism similar to glycerol, although, unlike the polycarboxylates, glycerol does not enhance TH when DAFP-1 is the only DAFP present. The carboxylate groups could bind one DAFP-1 molecule to another. The new aggregation of DAFP-1’s and enhancers could then block a larger surface area of the ice than that blocked by an individual DAFP-1 molecule. However, we can not rule out other mechanisms at this stage. For example, carboxylate groups may bind to a DAFP-1 molecule and thereby induce some slight structure change of the DAFP-1 molecule that permits the DAFP to better adsorb to ice.
The sequences and TH activities of the DAFP isoforms, DAFP-2 and DAFP-4, are similar to that of DAFP-1 [12, 25], and TmAFPs show high sequence identity to DAFPs [43]. This suggests that the addition of polycarboxylates or aminopolycarboxylates may also affect the TH activity of other DAFP isoforms and TmAFPs. Furthermore, the dependence of the efficiency of TH enhancers on their structural and physiochemical properties reported here should help to understand the mechanism of TH enhancers and design highly effective TH enhancers useful for certain applications of AFPs.
Acknowledgments
This study was supported in part by the start-up fund from California State University at Los Angeles and by the NIH-RIMI program at California State University, Los Angeles (P20 MD001824-01). We thank Dawn Verleye at University of Notre Dame.
Abbreviations
- AFP
Antifreeze protein
- TH
Thermal hysteresis activity
- DSC
Differential scanning calorimetry
- DAFP
Dendroides canadensis antifreeze protein
- EDTA
Ethylenediaminetetraacetate
- DTPA
Diethylenetriaminepentaacetate
- TTHA
Triethylenetetramine-N,N,N′,N″,N‴,N‴-hexaacetate
- NTA
Nitrilotriacetate
- BTCA
1,2,3,4-Butanetetra-carboxylate
- EGTA
Glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetate
- HAc
acetic acid
- CIT
Citrate
- Iso-CIT
Iso-citrate
- TCA
Tricarboxylate
- GTA
Glutarate
- HBA
β-Hydroxybutyrate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.DeVries AL, Wohlschlag DE. Freezing resistance in some Antarctic fishes. Science. 1969;163:1073–1075. doi: 10.1126/science.163.3871.1073. [DOI] [PubMed] [Google Scholar]
- 2.Duman JG. The role of macromolecular antifreeze in the darkling beetle, Meracantha contracta. J Comp Physiol B. 1977;115:279–286. doi: 10.1002/jez.1402010110. [DOI] [PubMed] [Google Scholar]
- 3.Tomchaney AP, Morris JP, Kang SH, Duman JG. Purification, composition, and physical properties of a thermal hysteresis “antifreeze” protein from larvae of the beetle Tenebrio molitor. Biochemistry. 1982;21:716–721. doi: 10.1021/bi00533a020. [DOI] [PubMed] [Google Scholar]
- 4.Tursman D, Duman JG, Knight CA. Freeze tolerance adaptations in the centipede, Lithobius forficatus. J Exp Zool. 1994;268:347–353. [Google Scholar]
- 5.Block W, Duman JG. Presence of thermal hysteresis producing antifreeze proteins in the antarctic mite, Alaskozetes antarcticus. J Exp Zool. 1989;250:229–231. [Google Scholar]
- 6.Duman JG. Subzero temperature tolerance in spiders: The role of thermal-hysteresis-factors. J Comp Physiol B. 1979;131:347–352. [Google Scholar]
- 7.Duman JG, Olsen TM. Thermal hysteresis protein activity in bacteria, fungi, and phylogenetically diverse plants. Cryobiology. 1993;30:322–328. [Google Scholar]
- 8.Urrutia ME, Duman JG, Knight CA. Plant thermal hysteresis proteins. Biochim Biophys Acta. 1992;1121:199–206. doi: 10.1016/0167-4838(92)90355-h. [DOI] [PubMed] [Google Scholar]
- 9.Graether SP, Gagne SM, Spyracopoulos L, Jia ZC, Davies PL, Sykes BD. Spruce budworm antifreeze protein: Changes in structure and dynamics at low temperature. J Mol Biol. 2003;327:1155–1168. doi: 10.1016/s0022-2836(03)00235-3. [DOI] [PubMed] [Google Scholar]
- 10.Graether SP, Kuiper MJ, Gagne SM, Walker VK, Jia Z, Sykes BD, Davies PL. β-helix structure and ice-binding properties of a hyperactive antifreeze protein from an insect. Nature. 2000;406:325–328. doi: 10.1038/35018610. [DOI] [PubMed] [Google Scholar]
- 11.Graham LA, Liou YC, Walker VK, Davies PL. Hyperactive antifreeze protein from beetles. Nature. 1997;388:727–728. doi: 10.1038/41908. [DOI] [PubMed] [Google Scholar]
- 12.Li N, Andorfer C, Duman J. Enhancement of insect antifreeze protein activity by solutes of low molecular mass. J Exp Biol. 1998;201:2243–2251. doi: 10.1242/jeb.201.15.2243. [DOI] [PubMed] [Google Scholar]
- 13.Andorfer CA, Duman JG. Isolation and characterization of cdna clones encoding antifreeze proteins of the pyrochroid beetle Dendroides canadensis. J Insect Physiol. 2000;46:365–372. doi: 10.1016/s0022-1910(99)00189-4. [DOI] [PubMed] [Google Scholar]
- 14.Graham LA, Davies PL. Glycine-rich antifreeze proteins from snow fleas. Science. 2005;310:461–461. doi: 10.1126/science.1115145. [DOI] [PubMed] [Google Scholar]
- 15.Lin FH, Graham LA, Campbell RL, Davies PL. Structural modeling of snow flea antifreeze protein. Biophys J. 2007;92:1717–1723. doi: 10.1529/biophysj.106.093435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeVries AL. Glycoproteins as biological antifreeze agents in Antarctic fishes. Science. 1971;172:1152–1155. doi: 10.1126/science.172.3988.1152. [DOI] [PubMed] [Google Scholar]
- 17.Raymond JA, DeVries AL. Adsorption inhibition as a mechanism of freezing resistance in polar fishes. Proc Natl Acad Sci USA. 1977;74:2589–2593. doi: 10.1073/pnas.74.6.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chao H, Houston ME, Hodges RS, Kay CM, Sykes BD, Loewen MC, Davies PL, Sonnichsen FD. A diminished role for hydrogen bonds in antifreeze protein binding to ice. Biochemistry. 1997;36:14652–14660. doi: 10.1021/bi970817d. [DOI] [PubMed] [Google Scholar]
- 19.Haymet ADJ, Ward LG, Harding MM, Knight CA. Valine substituted winter flounder ‘antifreeze’: Preservation of ice growth hysteresis. FEBS Lett. 1998;430:301–306. doi: 10.1016/s0014-5793(98)00652-8. [DOI] [PubMed] [Google Scholar]
- 20.Jia ZC, Davies PL. Antifreeze proteins: An unusual receptor-ligand interaction. Trends Biochem Sci. 2002;27:101–106. doi: 10.1016/s0968-0004(01)02028-x. [DOI] [PubMed] [Google Scholar]
- 21.Sicheri F, Yang DSC. Ice-binding structure and mechanism of an antifreeze protein from winter flounder. Nature. 1995;375:427–431. doi: 10.1038/375427a0. [DOI] [PubMed] [Google Scholar]
- 22.Cheng A, Merz KM., Jr Ice-binding mechanism of winter flounder antifreeze proteins. Biophys J. 1997;73:2851–2873. doi: 10.1016/S0006-3495(97)78315-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chou KC. Energy-optimized structure of antifreeze protein and its binding mechanism. J Mol Biol. 1992;223:509–517. doi: 10.1016/0022-2836(92)90666-8. [DOI] [PubMed] [Google Scholar]
- 24.Duman J. The inhibition of ice nucleators by insect antifreeze proteins is enhanced by glycerol and citrate. J Comp Physiol B. 2002;V172:163–168. doi: 10.1007/s00360-001-0239-7. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Duman JG. Antifreeze proteins of the beetle Dendroides canadensis enhance one another’s activities. Biochemistry. 2005;44:10305–10312. doi: 10.1021/bi050728y. [DOI] [PubMed] [Google Scholar]
- 26.Li N, Chibber BAK, Castellino FJ, Duman JG. Mapping of disulfide bridges in antifreeze proteins from overwintering larvae of the beetle Dendroides canadensis. Biochemistry. 1998;37:6343–6350. doi: 10.1021/bi972853i. [DOI] [PubMed] [Google Scholar]
- 27.Duman JG. Factors involved in overwintering survival of the freeze tolerant beetle, Dendroides canadensis. J Comp Physiol B. 1980;136:52–59. [Google Scholar]
- 28.Wu DW, Duman JG, Cheng CHC, Castellino FJ. Purification and characterization of antifreeze proteins from larvae of the beetle Dendroides canadensis. J Comp Physiol B. 1991;161:271–278. [Google Scholar]
- 29.Wu DW, Duman JG, Xu L. Enhancement of insect antifreeze protein activity by antibodies. Biochim Biophys Acta. 1991;1076:416–420. doi: 10.1016/0167-4838(91)90485-i. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Duman JG. A thaumatin-like protein from larvae of the beetle Dendroides canadensis enhances the activity of antifreeze proteins. Biochemistry. 2006;45:1278–1284. doi: 10.1021/bi051680r. [DOI] [PubMed] [Google Scholar]
- 31.Makhatadze GI. Thermal unfolding of proteins studied by calorimetry. Wiley-VCH Verlag GmbH & Co.; Weinheim: 2005. [Google Scholar]
- 32.Hansen TN, Baust JG. Differential scanning calorimetric analysis of antifreeze protein activity in the common mealworm, Tenebrio molitor. Biochim Biophys Acta. 1988;957:217–221. doi: 10.1016/0167-4838(88)90275-0. [DOI] [PubMed] [Google Scholar]
- 33.Lu M, Wang B, Li Z, Fei Y, Wei L, Gao S. Differential scanning calorimetric and circular dichroistic studies on plant antifreeze proteins. J Thermal Anal Cal. 2002;67:689–698. [Google Scholar]
- 34.Zhang DQ, Liu B, Feng DR, He YM, Wang JF. Expression, purification, and antifreeze activity of carrot antifreeze protein and its mutants. Protein Expr Purif. 2004;35:257–263. doi: 10.1016/j.pep.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 35.Ramlov HHv, DeVries AL, Wilson PW. Antifreeze glycoproteins from the Antarctic fish Dissostichus mawsoni studied by differential scanning calorimetry (DSC) in combination with nanolitre osmometry. Cryoletters. 2005;26:73–84. [PubMed] [Google Scholar]
- 36.Hansen TN, DeVries AL, Baust JG. Calorimetric analysis of antifreeze glycoproteins of the polar fish. Dissostichus mawsoni. Biochim Biophys Acta. 1991;1079:169–173. doi: 10.1016/0167-4838(91)90122-g. [DOI] [PubMed] [Google Scholar]
- 37.Zachariassen KE, DeVries AL, Hunt B, Kristiansen E. Effect of ice fraction and dilution factor on the antifreeze activity in the hemolymph of the cerambycid beetle Rhagium inquisitor. Cryobiology. 2002;44:132–141. doi: 10.1016/s0011-2240(02)00014-7. [DOI] [PubMed] [Google Scholar]
- 38.Zhang C, Zhang H, Wang L, Yao H. Validation of antifreeze properties of glutathione based on its thermodynamic characteristics and protection of baker’s yeast during cryopreservation. J Agric Food Chem. 2007;55:4698–4703. doi: 10.1021/jf070387q. [DOI] [PubMed] [Google Scholar]
- 39.Zachariassen KE, Husby JA. Antifreeze effect of thermal hysteresis agents protects highly supercooled insects. 1982;298:865–867. [Google Scholar]
- 40.Ringbom A. Complexation in analytical chemistry. Interscience; New York: 1963. [Google Scholar]
- 41.http://www.stanford.edu/~cpatton/xlsconstants.htm
- 42.Du N, Liu XY, Hew CL. Aggregation of antifreeze protein and impact on antifreeze activity. J Phys Chem B. 2006;110:20562–20567. doi: 10.1021/jp061969y. [DOI] [PubMed] [Google Scholar]
- 43.Graether SP, Sykes BD. Cold survival in freeze-intolerant insects. Eur J Biochem. 2004;271:3285–3296. doi: 10.1111/j.1432-1033.2004.04256.x. [DOI] [PubMed] [Google Scholar]