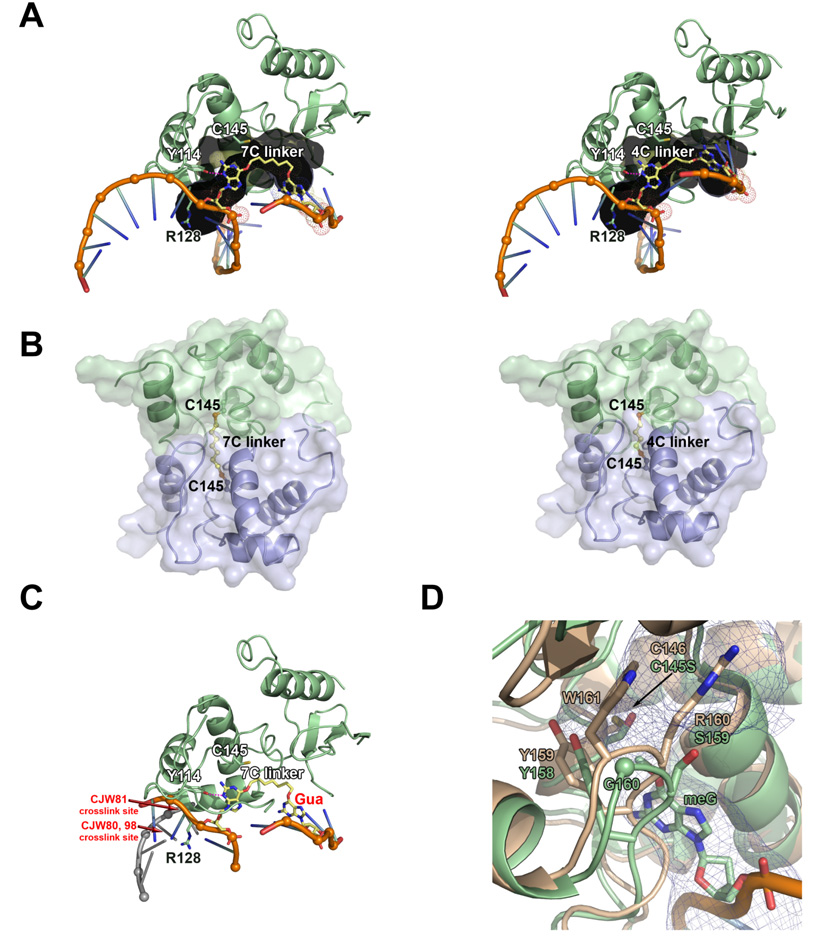
Figure 9. Model of O6-G-alkyl-O6-G interstrand cross-link repair by AGT.
(A 7-carbon (left) and 4-carbon (right) cross-linked substrate (yellow) modeled within the AGT active site channel (gray). (B) Model of AGT-AGT dimer resulting from repair of 7-carbon (left) and 4-carbon (right) cross-linked substrates. The protein-protein steric clash in the 4-carbon model could explain why this product is not observed experimentally. The N-terminal domain of AGT has been omitted from these models. (C) Model of AGT bound to 7-carbon cross-linked substrate and dsDNA, showing that repair of these cross-links by AGT likely disrupts the DNA double helix. Guanine labeled in red would have to be positioned at one of the cross-link sites (labeled in red) to maintain dsDNA. For clarity, helix 8 is not shown. (D) Ada-C structure (tan; pdb 1sfe) overlayed with AGT bound to DNA containing O6-methylguanine structure (green; pdb 1t38). The AGT active site channel is shown as a blue mesh.