Table 3.
Breadth and Scope of the Aldehyde Substratesa
 | |||||
|---|---|---|---|---|---|
| Ent | R | Temp, °C |
Time h |
Yield (%)b |
ee (%)c |
| 1 | C6H5 11 | rt | 2 | 74 | 32 |
| 2 | C6H5CH2 7 | rt | 2 | 80 | 71 |
| 3d | -10 | 24 | 68 | 82 | |
| 4 | C6H5CH2CH2 12 | rt | 2 | 78 | 60 |
| 5 | -10 | 24 | 64 | 84 | |
| 6 | 4-MeOC6H4CH2CH2 13 | rt | 2 | 77 | 81 |
| 7 | -10 | 24 | 64 | 86 | |
| 8 | 3,4-(MeO)2C6H3CH2 14 | rt | 2 | 76 | 72 |
| 9d | -10 | 24 | 68 | 84 | |
| 10 | 4-NO2C6H4CH2CH2 15 | rt | 2 | 74 | 75 |
| 11 | -10 | 24 | 75 | 82 | |
| 12 | 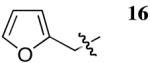 |
rt | 2 | 65 | 84 |
| 13 | -10 | 24 | 66 | 90 | |
| 14 | rt | 2 | 49 | 56 | |
| 15 | -10 | 24 | 50 | 85 | |
| 16 | Allyl 18 | rt | 2 | 49 | 80 |
| 17 | -10 | 24 | 58 | 90 | |
| 18 | (CH3)2CH 19 | rt | 24 | 74 | 0 |
For reaction conditions, see Supporting Information.
Isolated yield.
Determined by chiral HPLC.
4.0 equiv of TEMPO was used.
