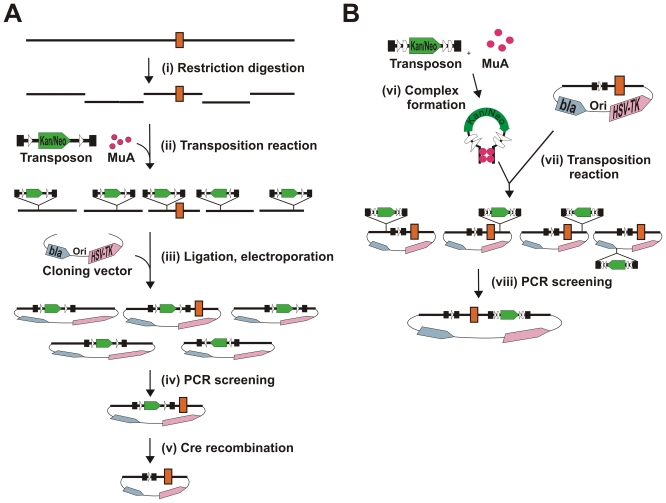
Figure 2. Flowchart for the construction of conditional knockout vectors.
Part A. (i) an entire BAC clone is initially digested with an appropriate restriction endonuclease, and (ii) the ensuing fragment pool is then used as a target for the first transposition reaction. (iii) The fragment pool is subsequently ligated into a suitable vector plasmid, and those clones that include transposon-containing BAC fragments are selected using both the transposon marker and vector marker. Next, (iv) a plasmid clone that contains a transposon insertion in a desired location is identified by a PCR screen with appropriate primers, one transposon specific and the other target specific. (v) The chosen plasmid is then introduced into an E. coli strain that expresses Cre recombinase. The selectable marker is eliminated by Cre recombination in vivo, leaving a single loxP site in the construction. Part B. The second transposition reaction (vi) using pre-assembled transposition complexes (vii) introduces into the construction a marker gene that is flanked by loxP and FRT sites. (viii) As above, a suitable clone is screened by PCR to identify a plasmid, which contains the second transposon inserted in a suitable location and orientation on the opposite side (to the first transposon) of the exon of interest. Genomic DNA is shown with a black line, and the orange rectangle denotes an exon. The transposons are shown with black bars featuring the Kan/Neo cassette (green arrow), loxP sites (white triangles), FRT sites (white pentagons), and transposon ends (black rectangles).