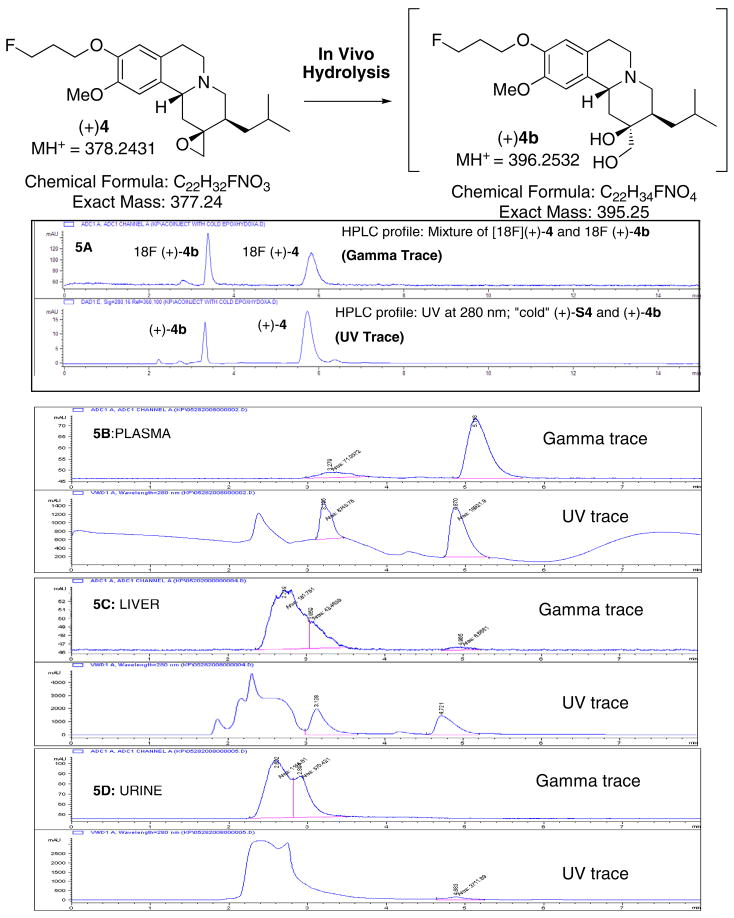
Fig. 5.
A proposed hydrolysis of epoxide (+)4 to (+)4b in vivo and in vitro is shown in this figure. It is likely that the epoxide ring of (+)4 was opened leading to the production of di-hydroxyl derivative, (+)4b. When “cold” (+)4 was treated with sodium hydroxide, it produced (+)4b. 5A: [18F](+)4 and [18F](+)4b were prepared and equal amounts of these two tracers were then mixed together (~1:1) and co-injected along with a cold mixture of (+)4 and (+)4b (~1:1) (HPLC condition: Phenomenex C18, acetonitrile/50 mM ammonium formate buffer, pH 4), 1 mL/min). After an injection of [18F](+)4 (37 MBq, 1 mCi) a rat was sacrificed and radioactive material was isolated from plasma, liver and urine samples. To the extracted samples, 5 μL of “cold mixture” was added and then injected into HPLC. The samples were eluted with the same solvent and column system (5B: plasma; 5C: liver; 5D: urine).