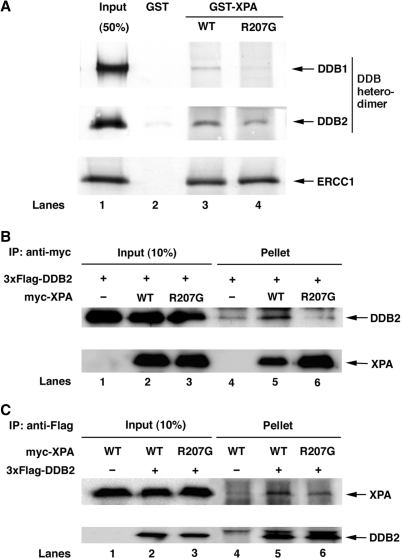
Figure 3.
R207G mutation reduces XPA binding to DDB. (A) Purified DDB heterodimer or in vitro translated ERCC1 was incubated with GST alone (lane 2), GST-XPA (lane 3) or GST-XPA(R207G) (lane 4) coupled to glutathione–sepharose 4B beads. The bound proteins were analyzed by western blotting with either anti-Flag followed by anti-mouse IgG conjugated with alkaline phosphatase (for DDB1 and DDB2) or streptavidin conjugated with alkaline phosphatase (for ERCC1). (B) and (C) myc-tagged XPA protein, wild-type or R207G mutant, was expressed in Tet-on U2OS/3xF-DDB2 cells in the presence of doxycycline and cell lysates were prepared after 40-h incubation. One or 0.3 mg of the lysates were incubated for 1.5 h with anti-FLAG M2 agarose (B) or anti-myc antibody followed by protein A/G plus agarose (C), respectively. Proteins retained on the beads were analyzed by western blotting using anti-Flag and anti-myc antibodies.