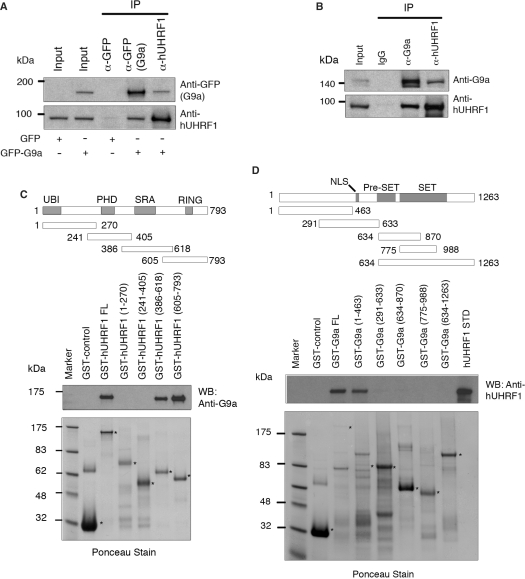
Figure 1.
Physical interaction of hUHRF1 and G9a. (A) Coimmunoprecipitation of hUHRF1 and G9a in cell extracts from COS-7 cells transfected with GFP control or GFP-G9a. The antibodies used for immunoprecipitation (IP) are indicated at the top of the panel. Western-blot analysis of immunoprecipitates was performed with antibodies as indicated. The input shows 2% of each lysate. (B) Coimmunoprecipitation of endogenous hUHRF1 and G9a in HEK293 cell extracts. HEK293 cells were synchronized to late S phase by serum starvation for 20 h and the subsequent release into 10% FBS-containing medium for 15 h before cell harvest. Antibodies used for immunoprecipitation and western blot are shown. The input represents 2% of each lysate. (C) Direct binding of hUHRF1 to G9a and mapping of the G9a-binding region on hUHRF1 using GST fusions of hUHRF1 fragments. Various domains of hUHRF1 are indicated along with a schematic presentation of the GST fusion constructs marked with amino acid numbers. The blot from GST pull-down assay was probed with anti-G9a antibody and stained with Ponceau solution to visualize the transferred proteins. Positions of fusion proteins are marked with asterisks. (D) Mapping of hUHRF1-binding region on G9a. Schematic diagram of the various GST-fusion constructs of G9a is shown with amino acid numbers. Western blot with anti-hUHRF1 antibody is shown along with the corresponding Ponceau-stained membrane.