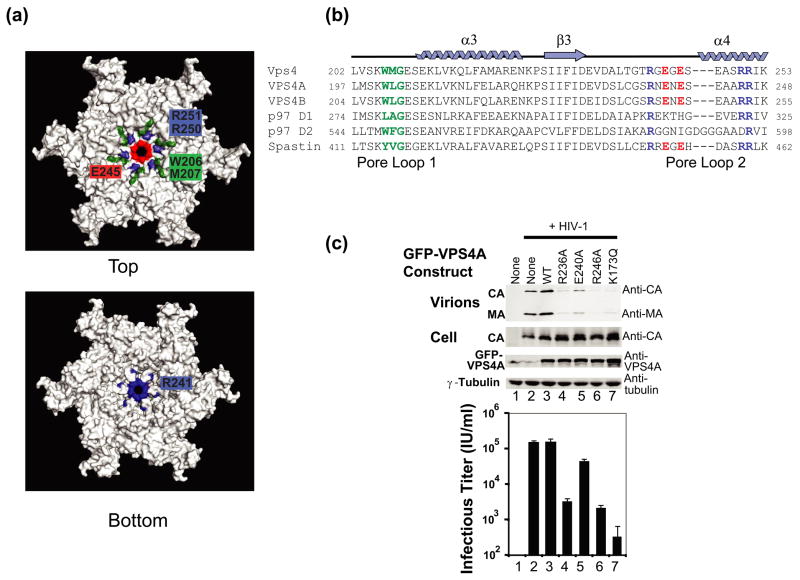
Figure 7.
VPS4A Pore Loop 2 residues are required for HIV budding.
(a) Two views of the p97 D1 Homology model for a hexameric ring of yeast Vps4ΔMIT in the ATPγS-bound state. Pore loop 1 residues (206WMG208) are shown in green, and a subset of the charged Pore Loop 2 residues are shown in blue (basic residues) or red (acidic residues).
(b) Sequence alignments highlighting Pore Loop 1 (green) and Pore Loop 2 (blue, basic, and red, acidic) residues from human and yeast VPS4 proteins. Equivalent residues from the related p97 and Spastin AAA ATPase cassettes are highlighted following the same color scheme (except for non-conservative changes).
(c) Vps4 Pore Loop 2 mutations dominantly inhibit HIV-1 release and infectivity. 293T cells were co-transfected with an empty vector control (lane 1) or with a proviral HIV-1 expression vector (lanes 2–7) together with the designated GFP-VPS4A expression constructs. The top two western blots show the levels of virion-associated CA and MA proteins released into the supernatant (Panel 1, Virion) or expressed in the cells (Panel 2, Cell). The bottom two western blots show cellular levels of GFP-VPS4A and of an γ-Tubulin control. The bottom panel shows vector titers in the supernatants under the different conditions (infectious units/ml). Note that expression of the different Pore Loop 2 mutant GFP-VPS4A constructs (lanes 3–6) or of the VPS4A ATPase mutant (lane 7, positive control) dominantly inhibited virion release and titers. Infectivity values are the mean of measurements from quadruplicate repeats and error bars indicate standard deviations.