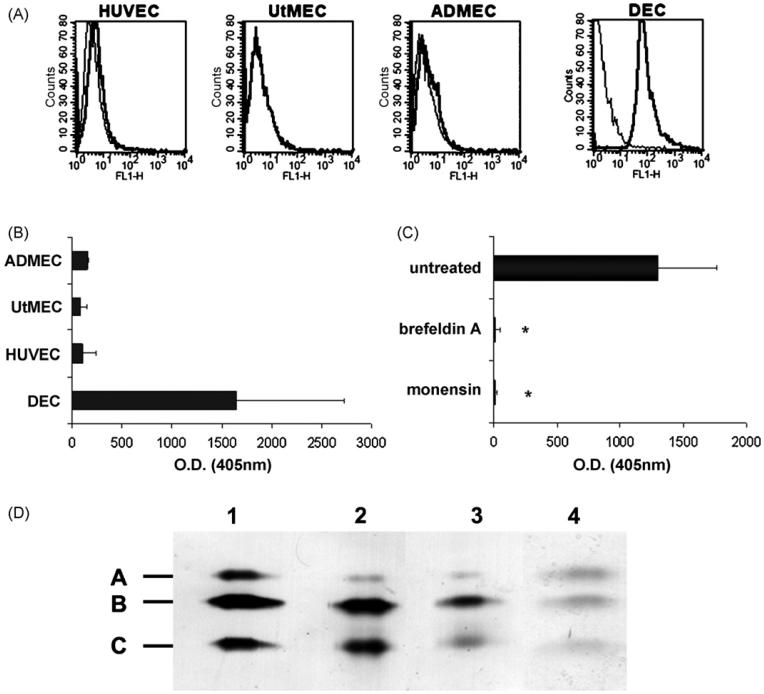
Fig. 4.
Analysis of ECs for surface expression and molecular characterization of C1q. (A) Cytofluorimetric analysis of C1q expression on freshly isolated ECs (DECs, UtMECs, ADMECs, HUVECs) incubated first with mAb for C1q (10 μg/ml) and then with FITC-conjugated F(ab′)2 fragment of goat anti-mouse Ig (1/50). (B) ELISA on ECs using DECs, UtMECs, ADMECs and HUVECs grown to confluence and incubated with mAb anti-human C1q (10 μg/ml) followed by AP-conjugated goat IgG anti-mouse IgG (1/6000). For details see Section 2. (C) ELISA on a confluent monolayer of DECs incubated with 5 μM monensin, 0.5 μM brefeldin A (Sigma-Aldrich) or medium alone for 24 h. The amount of C1q remaining on the cell surface was evaluated as indicated in B. *P < .01 versus untreated cells. (D) Western blot analysis of serum purified C1q from Quidel (track 1), and of C1q affinity-purified from cell supernatant (track 2) or from cell lysate (track 3). The blotted C1q bands were revealed using rabbit IgG anti-human C1q (1/1000) followed by AP-conjugated goat IgG anti rabbit IgG (1/20,000) (Sigma-Aldrich). C1q was also affinity purified from biotin-labeled DEC membranes and revealed with AP-conjugated streptavidine (1/4000) (Sigma-Aldrich) (track 4). The molecular weight of A-C chains of C1q were 29, 27 and 23 kDa respectively.