Table 4.
N-Aryl pyrrolidine synthesis
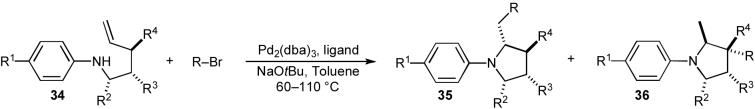 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | R | R1 | R2 | R3 | R4 | Ligand | Ratio 35:36 | dr | Yielda |
| 1 | 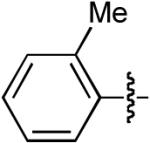 |
OMe | H | H | H | Dppb | 35:1 | - | 75% |
| 2 | 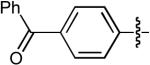 |
CN | H | H | H | Dppb | >100:1 | - | 86% |
| 3 | 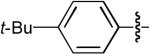 |
OMe | Me | H | H | Dppe | 10:1 | >20:1 | 66% |
| 4 | 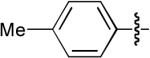 |
OMe | H | Ph | H | Dppe | 8:1 | 2:1 | 88% |
| 5 | 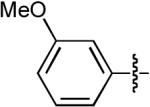 |
OMe | H | H | Ph | Dppe | 10:1 | >20:1 | 68% |
| 6 | 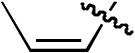 |
H | H | H | H | P(2-furyl)3 | >20:1 | - | 88% |
| 7 | 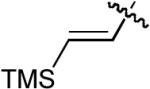 |
OMe | Me | H | H | P(2-furyl)3 | 7:1 | >20:1 | 79% |
| 8 | 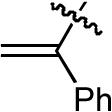 |
OMe | H | H | Ph | P(2-furyl)3 | >20:1 | 10:1 | 55% |
Conditions: 1.0 equiv amine, 1.1-2.0 equiv R-Br, 1.2 equiv NaOtBu, 1 mol % Pd2(dba)3, 2-4 mol % ligand, toluene (0.25 M), 60-110 °C.
