Table 8.
Synthesis of azabicyclo[3.3.0]octanes
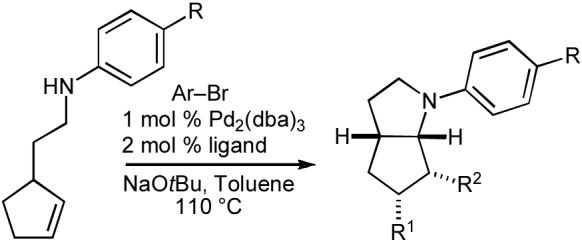 | |||||
|---|---|---|---|---|---|
| Entry | R | Ar | R1 | R2 | Yielda |
| 1b | CN | p-C6H4Me | H | Ar | 69% |
| 2b | CO2t-Bu | p-C6H4OMe | H | Ar | 58% |
| 3c | H | p-C6H4CN | Ar | H | 76% |
| 4c | Cl | p-C6H4CO2t-Bu | Ar | H | 71% |
Conditions: 1 equiv amine, 1.4 equiv ArBr, 1.2 equiv NaOtBu, 1 mol % Pd2(dba)3, 2 mol % ligand, toluene (0.25 M), 110 °C. All products were obtained with >20:1 dr.
Ligand = 2-diphenylphosphino-2′-(N,N-dimethylamino)biphenyl.
Ligand = P(t-Bu)2Me·HBF4.
