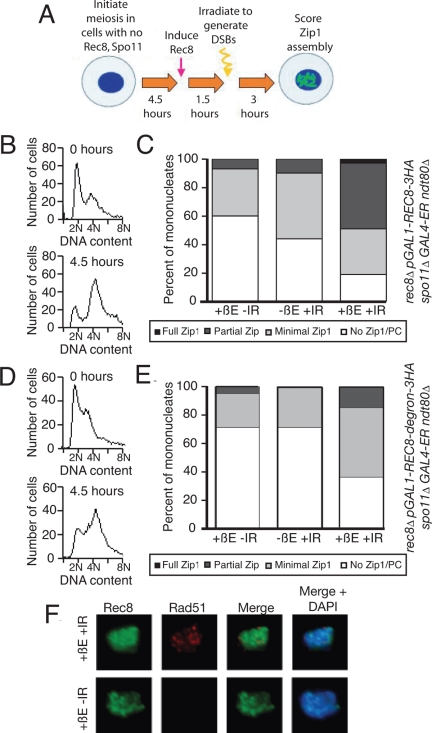
Figure 4.
Postreplicative Rec8 is sufficient for Zip1 assembly in the presence of DSBs. (A) The experimental scheme used in B–E. rec8::pGAL1-REC8-3HA spo11Δ rec8Δ GAL4-ER cells were induced to sporulate, allowed 4.5 h to progress through meiosis, and then treated with 1 μM β-estradiol (+βE). After another 1.5 h incubation in sporulation medium, cells were γ-irradiated with 20KRad (+IR) to induce DSBs. Cells were then kept in sporulation medium for another 3 h, when they were harvested and assayed for α-Zip1 and α-HA staining. A sample was also taken at 6.5 h to assay for DSBs by α-Rad51 staining. (B and C) rec8::pGAL1-REC8-3HA spo11Δ rec8Δ GAL4-ER ndt80Δ (A19800) cells were induced to sporulate and treated as described in A. At 9 h, cells with the indicated treatments were harvested, and chromosome spreads were assayed for Zip1 staining (C). DNA content was determined by flow cytometry analysis of cells harvested at 0 and 4.5 h of sporulation (B). (D and E) rec8::pGAL1-REC8-3HA-degron spo11Δ GAL4-ER ndt80Δ (A19798) cells were induced to sporulate and treated as described in A. At 9 h, cells with the indicated treatments were harvested, and chromosome spreads were assayed for Zip1 staining (E). DNA content was determined by flow cytometry analysis of cells harvested at 0 and 4.5 h of sporulation (D). (F) Examples of Rec8 and Rad51 staining in cells treated as described in A. At 6.5 h, cells were harvested and chromosome spreads were stained for Rec8, Rad51, and DNA. α-HA is shown in green, α-Rad51 in red, and DNA in blue.