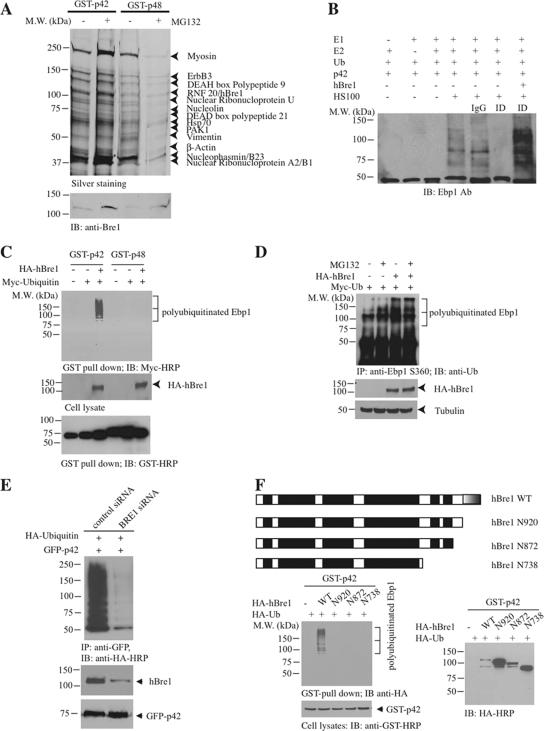
Figure 3.
hBre1 is an E3 ubiquitin ligase for Ebp1 p42. (A) hBre1 is an Ebp1-binding protein. Recombinant GST-p42 and GST-p48 proteins were incubated with cellular extract from DU145 cells, treated with MG132. After extensive washing, Ebp1-bound proteins were eluted with 1 M NaCl and analyzed by silver staining after SDS-PAGE. The bands selectively bound to Ebp1 were identified by proteomic analysis. The protein identity was labeled on the right side of the SDS-PAGE. RNF20/hBre1 strongly bound to p42 upon MG132 treatment. (B) hBre1 is required for p42 polyubiquitination in vitro. In vitro ubiquitination assay was conducted as described in Materials and Methods. Immunodepletion of hBre1 with its specific antibody but not control IgG abolished S-100's E3 ligase activity. Adding back hBre1 (1 μg) restored the E3 ubiquitin ligase activity. (C) hBre1 enhances transfected p42 polyubiquitination in vivo. GST-p42 and p48 were cotransfected with Myc-ubiquitin into HEK293 cells in the presence or absence of hBre1. Ebp1 was pulled down with glutathione beads and analyzed by immunoblotting with anti-Myc antibody. hBre1 selectively enhanced p42 but not p48 polyubiquitination (top). Verification of transfected proteins (middle and bottom). (D) Human Bre1 promotes endogenous p42 polyubiquitination. HA-ubiquitin and hBre1 were cotransfected into HEK293 cells, treated with 10 μM MG132 for 4 h. Ebp1 was immunoprecipitated with anti-phospho-S360 and analyzed with anti-ubiquitin. MG132 treatment enhanced polyubiquitination, and the maximal effect occurred when both hBre1 and MG132 were used. (E) Knocking down of hBre1 inhibits p42 polyubiquitination. HEK293 cells were transfected with GFP-p42 and Myc-ubiquitin, followed by control siRNA or hBre1 siRNA transfection. Ablation of hBre1 blocked p42 polyubiquitination. (F) hBre1 that contains Ring-finger domain is required for its E3 ligase activity. Schematic diagram of hBre1. Ring finger domain at the C terminus is indicated by half shaded box. The black boxes are the predicted coiled-coil domain. Predicted coiled-coil domains are shown as black boxes (top). Deletion of the Ring-finger domain crippled hBre1's E3 ligase activity (bottom left). Verification of transfected proteins (bottom).