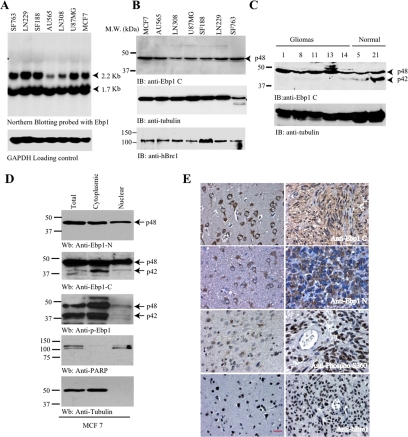
Figure 6.
Ebp1 p42 but not p48 is lost in human cancer cells through ubiquitination. (A) Northern blotting of Ebp1 in human cancer cell lines. Ebp1 encoded two mRNAs in various human cancer cell lines. (B) Ebp1 p42 expression is undetectable in various human cancer cells. Immunoblotting analysis of various human cancer cells with anti-Ebp1 antibody, which recognizes the C termini of both p48 and p42. Only p48 was detected (top). Similar levels of hBre1 and H2B were detected in all cells (third and bottom panels). (C) p42 is demonstrable in normal human brain samples, but it is degraded in human primary gliomas. The number on the top of the gel is the sample ID. (D) Subcellular fractionation of MCF7 cells. Three Ebp1 antibodies were characterized by immunoblotting with subcellular fractionated MCF7 cells (top to third panel). Identities if nuclear and cytosolic fractions were confirmed (fourth and bottom panels). (E) Immunohistochemistry on human primary gliomas. Both anti-Ebp1 N (specific for p48) and anti-Ebp1 C (recognizing both p42 and p48) revealed robust cytoplasmic staining and faint nuclear staining in neurons and astrocytes (left top and second panels). Anti-Ebp1 C staining displayed in both the cytoplasm and the nucleus in gliomas, whereas anti-Ebp1 N exclusively stained the cytoplasm of the neoplastic cells (right top and second panels). Normal neurons and astrocytes showed faint Ebp1 S360 phosphorylation. In contrast, glioblastoma displayed strong nuclear staining by anti-phospho-S360 (third panel). hBre1 specifically distributed in the nucleus of all cells (bottom). Bar, 10 μm.