Figure 2.
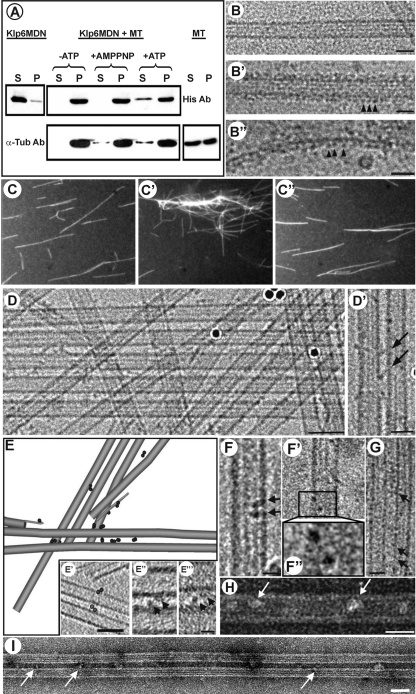
Evidence for Klp6MDN and Klp5/6FL cross-bridging of MTs. (A) Cosedimentation of Klp6MDN with MTs is ATP sensitive. Klp6MDN (2 μM) was incubated with Taxol-stabilized MTs (3 μM total tubulin) for 15 min at room temperature under various conditions: the absence of ATP, in the presence of 2 mM MgAMP-PNP, or in the presence of 2 mM MgATP. Samples were centrifuged for 30 min at 30,000 × g, and the pellets (P) were resuspended in SDS-buffer at a volume equal to the supernatants (S). Equal amounts of pellet and supernatant fractions were loaded on SDS gels and analyzed by Western blots. The top panel was probed with anti-His and the bottom panel with anti α-tubulin antibodies. (B) MT binding of Klp6MDN visualized by cryo-electron microscopy. (B) A rapidly frozen, ice-embedded, Taxol-stabilized MT. (B′) A similar MT after incubation with Klp6MDN for 10 min without ATP; motor-decorations (arrowheads) are evident on MT walls and on single protofilament extensions (B″). Bars, 25 nm. (C) Klp6MDN promotes ATP-sensitive MT bundling. Rhodamine-labeled MTs (C) were incubated with 0.5 μM Klp6MDN in the absence (C′) or presence (C″) of 1 mM MgATP. Samples were taken after 5 min, fixed with 0.2% glutaraldehyde, and then visualized by fluorescence light microscopy. (D) MT bundling in the presence of Klp6MDN visualized by cryo-EM. Without Klp6MDN most MTs were randomly distributed in the ice-layer (data not shown); in the presence of Klp6MDN, many MTs ran parallel to each other, forming bundles of up to ∼10 MTs (D). The distance between neighboring MTs within these bundles ranged from ∼10–13 nm. (D′) In some areas the gap between neighboring MTs was bridged by diagonal linkers (arrows). Dark circles are colloidal gold used for alignment of tilted views. Bars, 50 nm (D); 25 nm (D′). (E) Klp6MDN links between MTs were dimers when seen in 3D reconstructions. A 3D graphical model of a subvolume from a cryo-tomogram of ice-embedded MTs with Klp6MDN. The sample was imaged at 3° intervals over a tilt range of −63° to +72°. MTs and their protofilament extensions were modeled in gray, attached motors are shown as dark 4-nm discs. The majority of Klp6MDN particles occurred in pairs (circled in E′). E′–E‴ show 1-nm-thick tomographic slices of Klp6MDN dimers; E″ and E‴ show two motor heads (arrowheads) that bind adjacent MTs and are connected, most likely by their neck-domain. Bars, 50 nm (E′); 10 nm (E″ and E‴). (F–I) MT binding and bundling by Klp5/6FL visualized by cryo- (F and G) and negative staining (H and I) EM. Taxol-stabilized MTs were incubated with Klp5/6FL at a ratio of 1:2 plus 2 mM AMPPNP for 10 min. Klp5/6FL motors (arrows) bind to MTs (F–F″, H), often as dimers (F–F″). F″ is a 2× magnification of the two motor domains in the boxed area in (F′). Klp5/6FL also promotes MT bundle formation (G and I), whereby neighboring MTs seem to be connected by diagonal linkers (G, arrows). Bars, 50 nm (I); 25 nm (G and H); 12.5 nm (F and F′). Protein is black in B′–B″ and D–G and white in H and I due to different EM techniques used to prepare these images.