Figure 2.
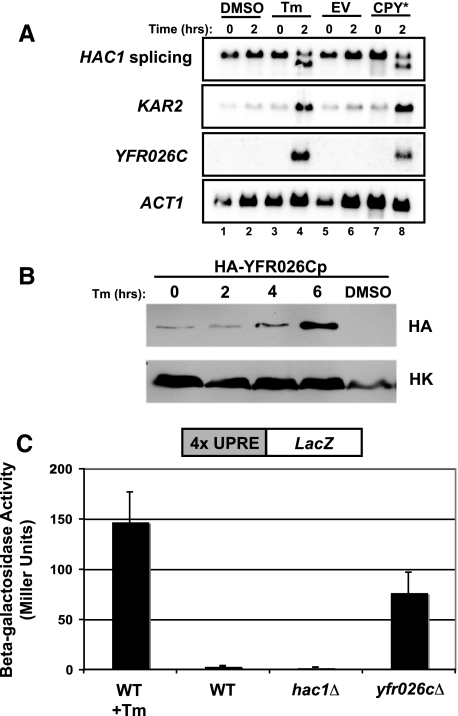
YFR026C is a novel gene (designated ULI1, see discussion), highly induced by UPR-L stress. (A) Northern blot to examine UPR-L gene induction by the ER stressors, tunicamycin (Tm; lanes 1–4) and CPY* (lanes 5–8). In lanes 1–4, wild-type cells (SM4460) were treated with either DMSO or 10 μg/ml Tm. Samples were harvested before (0 h) and after (2 h) treatment. In lanes 5–8, wild-type cells (SM4460) expressing empty vector (EV; pSM922) or CPY* (pSM2215) were harvested before (0 h) and after (2 h) galactose induction. Blots were probed to examine HAC1 splicing, KAR2 induction, and YFR026C induction. The ACT1 levels served as a loading control. (B) A Western blot for YFR026Cp protein levels was performed using extracts prepared from cells expressing genomic HA-tagged YFR026Cp (SM5295) under control of its endogenous promoter. Cultures were either untreated (0 h) or treated with 10 μg/ml Tm for 2, 4, and 6 h or DMSO for 6 h and harvested for protein at each time point. Blots were probed with anti-HA and anti-Hexokinase (HK) antibodies. (C) To examine whether lack of YFR026C causes induction of the UPR-L, yfr026cΔ (SM5476) cells expressing a UPRE-LacZ reporter (pPW344) were assayed for β-galactosidase activity. For comparison, the activity of wild-type (SM4460) cells alone (WT) or treated with 10 μg/ml Tm for 2 h before processing (WT + Tm), and hac1Δ cells (SM5382), all expressing a UPRE-LacZ reporter (pPW344), are included as controls. The data reflect an average of three independent experiments; error bars, 1 SD.