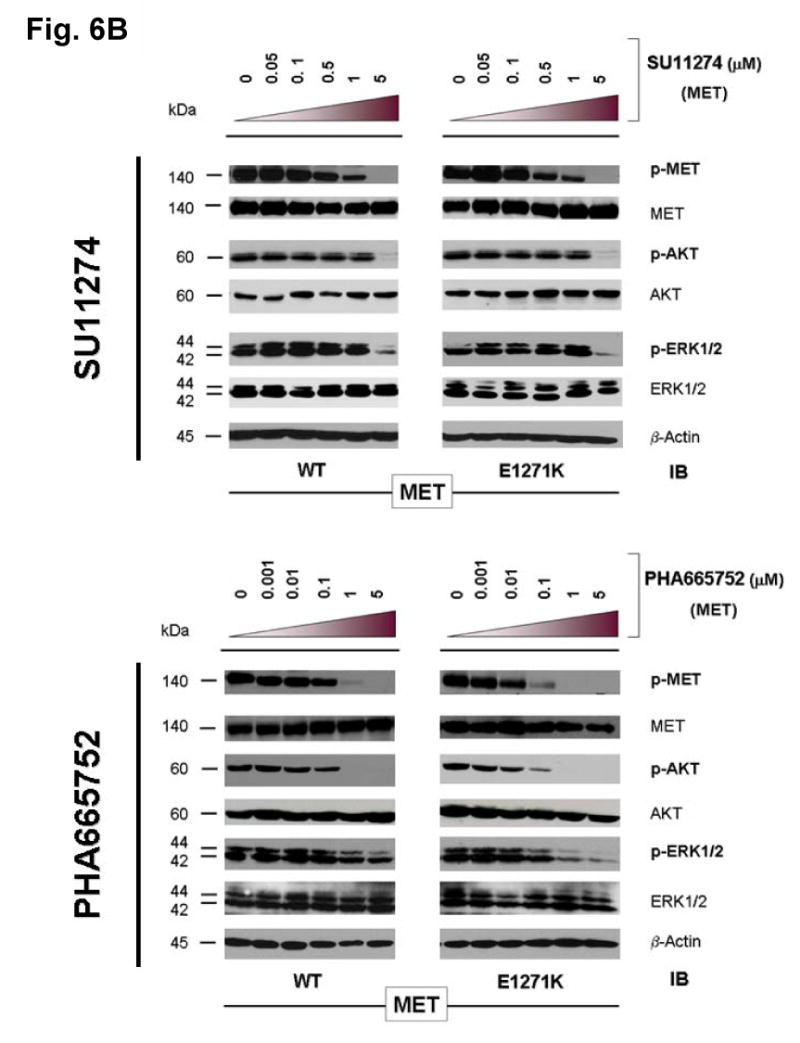
Figure 6. Mutational disruption of the conserved E1271-R1345 ion pair in MET kinase salt bridge causes inhibitor specific modulation of sensitivity to SU11274 (unchanged) and PHA665752 (more sensitive).
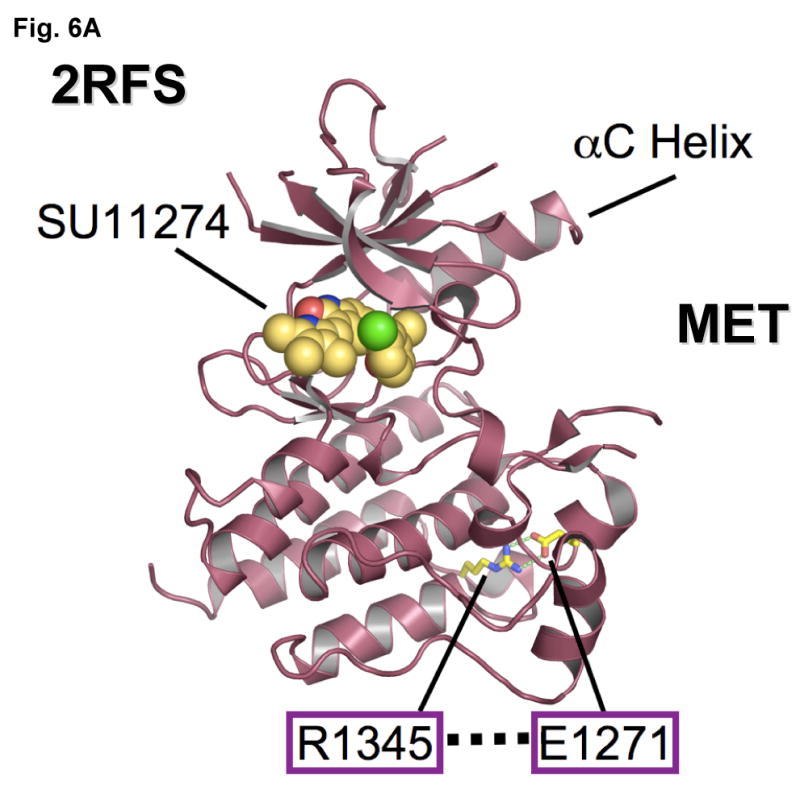
(A) MET kinase domain crystal structure (PDB accession code: 2RFS) (Bellon et al., 2008) highlighting the salt bridge between E1271 and R1345. Crystal structure was solved in complex with SU11274, shown. The conserved Glu-Arg ion pair is shown in stick format, with oxygen atoms colored red, nitrogens colored blue and carbon colored yellow. Figure was prepared using the program PYMOL (www.pymol.org).
(B) Stable COS-7 transfects expressing E1271K mutant MET were cultured in 0.5% BSA-containing serum-free media for 16 hours, then incubated with increasing concentrations of the MET inhibitors SU11274 (top) and PHA665752 (bottom) as indicated, in the presence of HGF stimulation (50ng/ml). Whole cell lysates were extracted for immunoblotting using antibodies against p-MET [Y1234/Y1235], MET, p-AKT, AKT, p-ERK1/2, ERK1/2 and β-actin. Wild-type MET expressing COS-7 tranfectant cells were included as control. E1271K mutation of MET increased the sensitivity of MET kinase phosphorylation inhibition by PHA665752.
