Fig. 2.
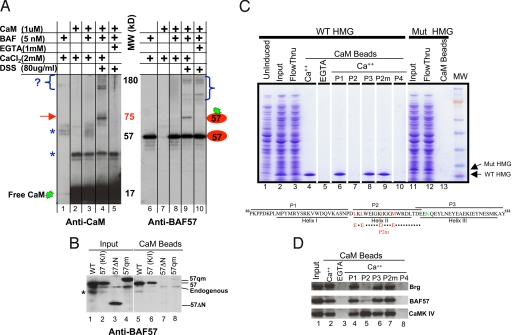
CaM binds the HMG domain of BAF57. (A) In vitro cross-linking assay. CaM was cross-linked to the BAF complex and the reaction products resolved by SDS/PAGE before being probed sequentially with antibodies against CaM (Left) and BAF57 (Right). Brackets denote crosslinking between CaM and unknown proteins (Left), or between BAF57 and its neighboring subunits (Right). Arrow, crosslinking between CaM and BAF57. Asterisks, nonspecific immunoreactivity. (B) Anti-BAF57 Western blotting showing that CaM beads captured BAF57 (WT) and the BAF57 point mutant defective in DNA binding [57(K/I)], but not the deletion mutant (57 ΔN) lacking the N-terminal 133 residues or the quadruple point mutant (57qm) bearing substitutions in hydrophobic residues as depicted in C. WT and mutant BAF57, Flag-tagged to distinguish them from endogenous BAF57, were transiently overexpressed in 293T cells; 293T cells were used because they are highly transfectable. Asterisk, nonspecific reactivity or BAF57 degradation product. (C) Coomassie blue-stained gel showing that CaM beads pulled down WT BAF57 HMG domain (lanes 1–5), but not the point mutant (lanes 11–13) from bacterial lysates, and the effects of various peptides (100 μM) on CaM-BAF57 HMG interaction (lanes 6–10). Illustrated at the bottom are the WT and mutant BAF57 HMG boxes together with the corresponding peptides. The hydrophobic residues involved in CaM binding and the lysine residue essential for DNA binding are highlighted in red and green, respectively. P2m, mutant P2 bearing the quadruple point mutations. (D) Western blots showing the effects of various peptides (100 μM) on CaM-BAF and CaM-CaMK IV interactions in thymocyte NE where CaMK IV is readily detectable. The peptides were added to the NE together with CaM beads.