Figure 1.
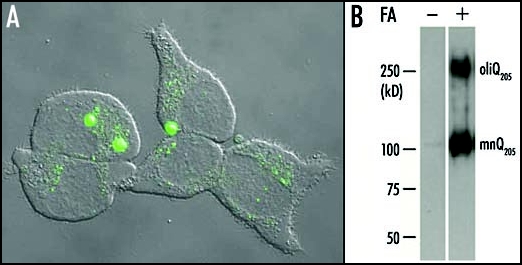
Aggregation of Q205 in PC12 cells. (A) A 24 h induction by ponasterone A was suffisant to induce the formation of large aggregates containing EGFP-Q205 in the cell line PC12-EGFP-Q205. Each cell contained a small number (one to three usually) of large aggregates and showed a very low level of diffuse cytoplasmic fluorescence. This suggested that most of the soluble recombinant protein had been recruited to large aggregates. However, a number of cells displayed either a multitude of small aggregates, or a high level of diffuse cytoplasmic fluorescence with no evidence of aggregation. These differences are probably related to differences in the time-course of aggregation in different cells. (B) Purified inclusions from the cell line PC12-Q205 were either treated or not with formic acid (FA), boiled in SDS/2-ME and submitted to electrophoresis through 7.5% polyacrylamide. After transfer to nitrocellulose, the proteins were stained with the 1C2 antibody. In the absence of formic acid treatment, no signal was detected. After treatment with 90% formic acid for one hour at 37°C, electrophoresis revealed a strong band corresponding to a monomer (mnQ205) with an apparent molecular mass of ∼100 kDa; the deduced molecular mass is 36,340, but expanded polyQ reduces mobility in gel electrophoresis. A second band with a considerably higher molecular mass was clearly stained and corresponded to an oligomer (oligQ205). Reproduced from Iuchi et al.30
