Figure 1.
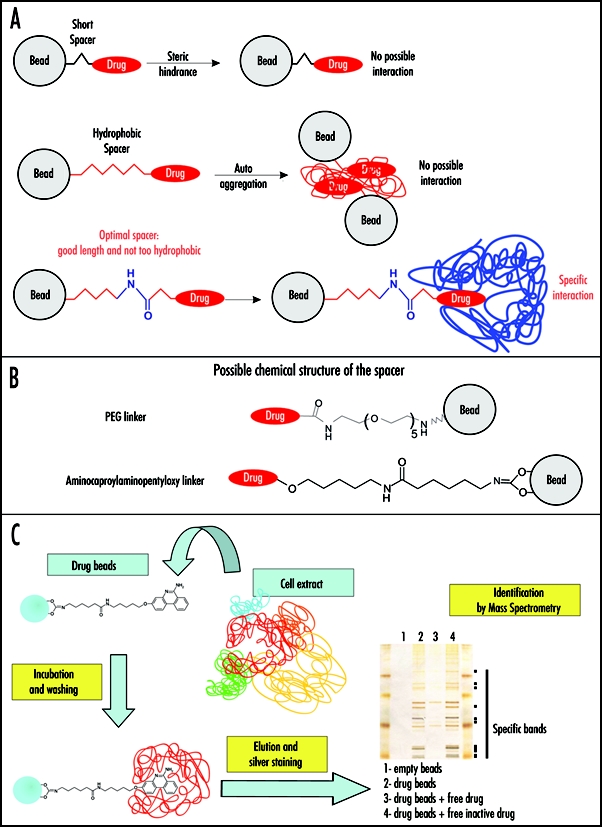
Antiprion drugs as baits in affinity chromatography on immobilized compounds. (A) The structure of the linker connecting the drug to the matrix (Sepharose bead) is crucial. A short spacer can prevent interaction with cellular targets because of steric hindrance (upper scheme). If the spacer is too hydrophobic (depicted in red in the middle panel), it can lead to its auto aggregation thus also preventing target binding. In addition, a hydrophobic linker can generate unspecific hydrophobic interactions with macromolecules from cell lysates. An optimal spacer (lower panel) should be only mildly hydrophobic (hydrophilic part depicted in blue) and long enough to avoid steric hindrance. In this case, specific interactions can occur, as long as the covalent link of the linker to the drug does not affect its antiprion activity too strongly. (B) Possible linker chemical structures are depicted. Polyethylene glycol (PEG) (upper panel) and amino caproylaminopentyloxy linker (lower panel). (C) Affinity chromatography purification on immobilized 6AP. In the example given, the antiprion drug 6AP was covalently liked to Sepharose beads via an amino caproylaminopentyloxy linker. Cell extracts were then incubated with this matrix. After extensive washing, the affinity matrix bound cellular components were analyzed by SDS PAGE followed by silver staining. The interacting proteins were identified by mass spectrometry.
