Abstract
The photoprotective role of the universal violaxanthin cycle that interconverts violaxanthin (V), antheraxanthin (A), and zeaxanthin (Z) is well established, but functions of the analogous conversions of lutein-5,6-epoxide (Lx) and lutein (L) in the selectively occurring Lx cycle are still unclear. We investigated carotenoid pools in Lx-rich leaves of avocado (Persea americana) during sun or shade acclimation at different developmental stages. During sun exposure of mature shade leaves, an unusual decrease in L preceded the deepoxidation of Lx to L and of V to A+Z. In addition to deepoxidation, de novo synthesis increased the L and A+Z pools. Epoxidation of L was exceptionally slow, requiring about 40 d in the shade to restore the Lx pool, and residual A+Z usually persisted overnight. In young shade leaves, the Lx cycle was reversed initially, with Lx accumulating in the sun and declining in the shade. De novo synthesis of xanthophylls did not affect α- and β-carotene pools on the first day, but during long-term acclimation α-carotene pools changed noticeably. Nonetheless, the total change in α- and β-branch carotenoid pools was equal. We discuss the implications for regulation of metabolic flux through the α- and β-branches of carotenoid biosynthesis and potential roles for L in photoprotection and Lx in energy transfer to photosystem II and explore physiological roles of both xanthophyll cycles as determinants of photosystem II efficiency.
It has long been recognized that photosynthesis in plants must resolve two conflicting requirements, the need to ramp up maximum light-harvesting efficiency in dim light and to wind back to lower efficiency when light is in excess, in order to maintain high rates of growth and productivity in varying light environments (Björkman, 1981; Pearcy, 1990). A wealth of research has established that plants adjust through an array of morphological and molecular events that confer photoprotection, mitigate and repair photoinactivation of PSII, and facilitate acclimation of the photosynthetic apparatus over different time scales in response to variable light regimes in wild plants, crops, and algae (Osmond et al., 1999; Demmig-Adams et al., 2006). In the context of the light reactions, low light acclimation optimizes light harvesting and energy transfer to the photosystems, particularly PSII, via enlarged functional antennae, accumulation of accessory light-harvesting pigments, and down-regulation of unnecessary competing photoprotective processes. High light acclimation involves increased photoprotection and photorepair, downsized antennae, fewer photosystems, and sometimes changes in the PSI to PSII stoichiometry (Osmond et al., 1999; Förster et al., 2005). Along with their function in energy transfer to the photosynthetic reaction centers as accessory pigments to chlorophylls, the xanthophyll pigments violaxanthin (V), antheraxanthin (A), and zeaxanthin (Z) play a central role in these transformations of the photosynthetic apparatus, especially in thermal energy dissipation and detoxification of reactive oxygen species.
Two xanthophyll cycles are now known in terrestrial plants, the lutein epoxide cycle (Lx cycle) based on interconversions of lutein-5,6-epoxide (Lx) and lutein (L) synthesized from α-carotene (α-C), and the violaxanthin cycle (V cycle) based on the interconversions of V and A+Z synthesized from β-carotene (β-C; García-Plazaola et al., 2007). Presumably, both cycles are catalyzed by the same enzymes, violaxanthin epoxidase (VDE) for deepoxidation in high light and zeaxanthin epoxidase (ZE) for the reverse reactions in low light or darkness (Latowski et al., 2004). Rediscovery of the Lx cycle in the parasitic angiosperm Cuscuta reflexa (Bungard et al., 1999) has led to growing interest in differing manifestations of this cycle in terrestrial plants and its relationships to the apparently universal V cycle (Demmig-Adams, 1998). A complete Lx cycle seems to function on a daily basis in both C. reflexa and the mistletoe Amyema miquelii (Matsubara et al., 2001), even though Lx conversion to L is sometimes slower than V to A+Z and dark recovery of Lx is usually slower than that of V. Intriguingly, in shade leaves of Inga sapindoides, high concentrations of Lx were seemingly irreversibly converted to L on exposure to strong light, in marked contrast to the co-occurring, fully reversible V cycle (Matsubara et al., 2005). Similar responses have been found in other woody plants with long-lived leaves in deeply shaded canopies, including Mediterranean oaks (Quercus spp.; García-Plazaola et al., 2003), sweet bay laurel (Laurus nobilis), and avocado (Persea americana; Esteban et al., 2007, 2008). This response type is known as a truncated Lx cycle (García-Plazaola et al., 2007).
The functions attributed to the Lx cycle were initially based on structural analogies between Lx and A and between L and Z (Bungard et al., 1999; Pogson and Rissler, 2000; Matsubara et al., 2001). With increased evidence for the possible role of L in photoprotection (Pogson et al., 1996, 1998; Lokstein et al., 2002; Dall'Osto et al., 2006), additional functional analogies emerged. Furthermore, recent in vitro reconstitution studies with light-harvesting complex proteins and purified pigments also support a spatial overlap of the cycles, as some pigment-binding sites can be occupied by either α- or β-xanthophylls (Matsubara et al., 2007). An attractive hypothesis is that photoconversion of Lx to L might sustain or enhance photoprotection associated with the V cycle (Demmig-Adams and Adams, 1992; Niyogi, 2000). In support of this view, it has been demonstrated in leaves of Quercus rubra and in leaflets of Inga marginata that increasing amounts of photoconverted L, which persist even when A and Z are epoxidized to V, were associated with faster engagement and higher levels of nonphotochemical quenching (NPQ) of chlorophyll fluorescence (García-Plazaola et al., 2003; Matsubara et al., 2008). Furthermore, evidence from mammalian eye research as well as from plants suggests that L also acts as a highly efficient reactive oxygen species scavenger (Kim et al., 2006; Johnson et al., 2007).
Broader issues, such as the roles of short-term dynamics of the two cycles in relation to long-term processes of shade and sun acclimation and in relation to leaf development and age, are poorly understood. Nonfruiting shoots of avocado trees constitute a very suitable model system in which to address these issues. Long-lived leaves of shade-grown avocado contain some of the highest levels of Lx thus far recorded (Esteban et al., 2007; García-Plazaola et al., 2007) and have two to four flushes of leaf initiation per year that exhibit a form of delayed greening in which leaf expansion precedes increases in stomatal conductance, chlorophyll content, and CO2 assimilation. Expanding leaves remain sinks for up to 1 month until they reach about 70% to 80% of full expansion (Schaffer et al., 1991), and stomata do not become fully functional until leaves attain 90% of full expansion (Scholefield and Kriedemann, 1979). However, shoots also retain old leaves through several flushes, and leaves from the previous season contribute significantly to total plant carbon gain (Liu et al., 2002), with photosynthesis rates up to 50% of those in new, fully expanded leaves (Heath et al., 2005). These properties offer an array of opportunities for new research into the concurrent operation of the two xanthophyll cycles.
Since there have been very few studies of these complex responses, we carried out a series of short- and long-term light treatments that are likely to reflect what leaves may experience in natural environments, with the aim to gain further insight into the physiological relevance of the Lx and V cycles under those circumstances. Four types of acclimation experiments were undertaken in this study. First, short-term acclimation from shade to sun addressed fast responses to a drastic increase in the light environment, simulating a prolonged sun fleck in shaded mature leaves or exposure to a bright sunny day in young leaves that had emerged during a prolonged overcast (shaded) growth period. These experiments revealed an unexpected loss of L prior to deepoxidation of Lx and V and a reverse Lx cycle in young leaves. Second, long-term acclimation of sun leaves to prolonged shade simulated normal processes of shading by further growth of outer canopy leaves. These treatments established the very slow accumulation of Lx in avocado leaves. Third, sequential sun exposures of mature leaves over several days, followed by continuous shade, were applied to simulate successive prolonged sun flecks, mimicking stochastic canopy disturbance during severe weather events, which confirmed many responses in the above experiments, particularly the very slow epoxidation of L to Lx in prolonged shade. Fourth, long-term acclimation of young and mature leaves to sun was examined. These experiments simulated sudden changes to canopy architecture as experienced during pruning and extended our understanding of the comparative rates and magnitude of Lx and V cycle engagement. We discuss the short-and long-term kinetics of both cycles in avocado leaves of different ages during acclimation, with particular attention to the stoichiometric relationships between xanthophyll and carotenoid pools and changing PSII efficiency.
RESULTS
Table I profiles baseline control measurements (at 6 am) of carotenoid pool sizes on a chlorophyll basis in shade- and sun-acclimated avocado leaves in relation to age (size) that provide a foundation for our investigations of light responses in the Lx and V cycles in these plants. Young leaves had 50% lower chlorophyll content than mature leaves (Table I) but total carotenoid pools on a chlorophyll basis were similar in both leaf categories, so that total carotenoids per leaf area were substantially larger in mature leaves. The chlorophyll-based concentration of neoxanthin (N), an important structural component of antenna Lhcs, was remarkably stable in both sun and shade leaves and about one-third lower in sun leaves (Tables I and II). The V+A+Z pool was noticeably larger in sun leaves that also contained higher residual levels of A and Z with age. In contrast, the Lx pool increased with age in shade leaves and to a lesser extent in sun leaves, whereas the L pool declined in mature shade leaves but was more stable in sun leaves. Pool sizes of α-C increased markedly with age in shade leaves, β-C pools increased in sun leaves, and chlorophyll a/b ratios were consistently lower in shade leaves, as observed in other species (Thayer and Björkman, 1990; Krause et al., 2001). Photosynthetic efficiency estimated from maximum photochemical efficiency of PSII in the dark-adapted state (Fv/Fm) was slightly lower in sun leaves than in shade leaves, and generally PSII efficiency was highest in mature leaves. Mature shade leaves sampled at the start and at the conclusion of the 3-month experimental period showed stable chlorophyll concentration on a leaf area basis and a trend toward increased total carotenoid concentration on a chlorophyll basis (Table II). The fact that there was no loss of chlorophyll confirmed that mature leaves did not senesce in long-term experiments.
Table I.
Light- and age-related pigment composition of young and mature avocado leaves acclimated to sun or shade
Leaves showed distinctive light-dependent pigment profiles in response to up to 38 d of sun and more than 43 d of shade that vary with leaf length, which was used as an indication of age (young, y1 and y2; mature, m). Total carotenoid (ΣCar; mmol mol−1 chlorophyll) and total chlorophylls (ΣChl; μmol chlorophyll a+b m−2) showed a trend to higher pigment levels in mature leaves. Z was not detected (n.d.) in shade leaves without a history of sun exposure, and base levels of Z and A persisted in sun-acclimated leaves. Photosynthetic capacity based on PSII efficiency (Fv/Fm) was estimated from chlorophyll fluorescence. Samples were taken at 6 am (experiments 2–5) between December 17, 2006 and January 31, 2007. Values are means ± se (n = 4).
| Leaf Age/Size | N | V | A | Z | Lx | L | α-C | β-C | α-C/β-C | Chlorophyll a/b | ΣCar | ΣChl | Fv/Fm |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shade leaves | |||||||||||||
| y1, 6–7 cm | 34.3 ± 0.4 | 53.3 ± 1.1 | 2.7 ± 0.3 | n.d. | 1.7 ± 0.6 | 138.5 ± 0.4 | 23.9 ± 0.5 | 48.6 ± 0.7 | 0.48 ± 0.02 | 2.62 ± 0.04 | 306 ± 2 | 0.15 ± 0.01 | 0.73 ± 0.01 |
| y2, 11–13 cm | 35.1 ± 1.4 | 53.0 ± 2.1 | 6.0 ± 0.3 | n.d. | 2.4 ± 0.1 | 140.2 ± 3.7 | 27.6 ± 2.1 | 48.4 ± 1.7 | 0.57 ± 0.04 | 2.49 ± 0.03 | 313 ± 10 | 0.16 ± 0.01 | 0.75 ± 0.02 |
| y2, 11–13 cm | 33.4 ± 0.2 | 55.6 ± 1.5 | 7.1 ± 0.5 | 3.3 ± 0.4 | 2.6 ± 0.1 | 140.0 ± 1.9 | 14.7 ± 0.9 | 52.4 ± 1.0 | 0.28 ± 0.02 | 2.65 ± 0.01 | 309 ± 4 | 0.35 ± 0.03 | 0.76 ± 0.01 |
| m, 18–25 cm | 39.4 ± 0.5 | 43.2 ± 0.9 | 2.5 ± 0.5 | n.d. | 19.2 ± 1.4 | 119.1 ± 2.5 | 73.2 ± 0.6 | 42.2 ± 0.7 | 1.74 ± 0.04 | 2.65 ± 0.05 | 339 ± 3 | 0.38 ± 0.02 | 0.79 ± 0.00 |
| m, 18–25 cm | 34.7 ± 0.5 | 36.6 ± 1.2 | 0.9 ± 0.1 | 0.8 ± 0.3 | 16.6 ± 1.2 | 113.2 ± 2.7 | 51.5 ± 1.8 | 42.4 ± 1.2 | 1.22 ± 0.07 | 2.81 ± 0.07 | 297 ± 4 | 0.50 ± 0.02 | 0.80 ± 0.01 |
| Sun leaves | |||||||||||||
| y1, 6–7 cm | 21.6 ± 0.7 | 62.0 ± 5.2 | 7.5 ± 0.4 | 4.4 ± 0.3 | 3.9 ± 0.4 | 138.1 ± 1.9 | 4.0 ± 0.2 | 50.9 ± 1.9 | 0.08 ± 0.00 | 2.89 ± 0.08 | 292 ± 8 | 0.15 ± 0.01 | 0.69 ± 0.03 |
| y2, 11–13 cm | 24.6 ± 0.5 | 49.0 ± 1.4 | 7.6 ± 0.3 | 5.4 ± 0.4 | 3.4 ± 0.3 | 146.8 ± 3.2 | 5.3 ± 0.5 | 52.5 ± 12.7 | 0.14 ± 0.05 | 2.99 ± 0.07 | 295 ± 18 | 0.19 ± 0.02 | 0.75 ± 0.00 |
| m, 18–25 cma | 28.7 ± 0.4 | 65.6 ± 3.1 | 10.9 ± 1.3 | 8.0 ± 0.7 | 10.9 ± 1.7 | 144.2 ± 3.3 | 22.9 ± 1.4 | 88.2 ± 2.0 | 0.26 ± 0.02 | 3.50 ± 0.07 | 370 ± 9 | 0.46 ± 0.02 | 0.73 ± 0.01 |
| m, 18–25 cmb | 25.0 ± 4.2 | 90.4 ± 8.5 | 10.5 ± 1.7 | 11.2 ± 1.0 | 10.5 ± 1.3 | 169.4 ± 8.8 | 14.0 ± 0.4 | 84.5 ± 1.0 | 0.17 ± 0.01 | 3.46 ± 0.02 | 413 ± 22 | 0.35 ± 0.03 | 0.72 ± 0.01 |
Young shade leaves that grew into mature sun leaves.
Mature shade leaves acclimated in 39 d of sun.
Table II.
Long-term pigment pool stability of mature avocado shade leaves
Mature shade leaves of various ages fully expanded less than 45 d earlier (upper canopy) and fully expanded more than 45 d to up to 2 years earlier (lower canopy) showed no signs of senescence-related loss or breakdown of total carotenoids (ΣCar; mmol mol−1 chlorophyll) or total chlorophylls (ΣChl; μmol chlorophyll a+b m−2). Z was not detected (n.d.), and traces of A persisted only in leaves that were sun acclimated for 30 d prior to 43 d of shade. Samples were taken at 6 am between November 6, 2006 and January 30, 2007. Values are means ± se (n = 4).
| Mature Leaves | N | V | A | Z | Lx | L | α-C | β-C | α-C/β-C | Chlorophyll a/b | ΣCar | ΣChl |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| >45 d | 42.0 ± 0.2 | 28.1 ± 0.2 | n.d. | n.d. | 59.8 ± 1.0 | 103.3 ± 2.2 | 65.1 ± 1.1 | 38.3 ± 1.4 | 1.71 ± 0.06 | 2.51 ± 0.01 | 335 ± 2 | 0.46 ± 0.02 |
| <45 d | 39.5 ± 0.2 | 32.9 ± 1.3 | n.d. | n.d. | 27.8 ± 1.3 | 118.0 ± 2.6 | 63.6 ± 1.1 | 35.4 ± 1.4 | 1.80 ± 0.06 | 2.79 ± 0.06 | 317 ± 6 | 0.40 ± 0.05 |
| >45 da | 36.6 ± 3.9 | 30.1 ± 3.9 | 1.3 ± 0.1 | n.d. | 47.6 ± 3.2 | 97.5 ± 11.3 | 40.0 ± 6.8 | 42.4 ± 5.3 | 0.97 ± 0.18 | 2.70 ± 0.06 | 296 ± 27 | 0.55 ± 0.05 |
| <45 dab | 43.3 ± 5.9 | 41.3 ± 6.4 | 2.4 ± 0.6 | n.d. | 25.7 ± 1.7 | 119.5 ± 16.3 | 65.7 ± 10.5 | 48.2 ± 4.4 | 1.34 ± 0.10 | 2.67 ± 0.04 | 346 ± 44 | 0.65 ± 0.08 |
Sun acclimated for 30 d prior to 43 d of shade.
Sun-emerged leaves that grew into mature leaves in the shade.
Mature Shade Leaves Exposed Short Term to Sunlight Showed Distinct Effects on the Lx and V Cycle Pigment Pools
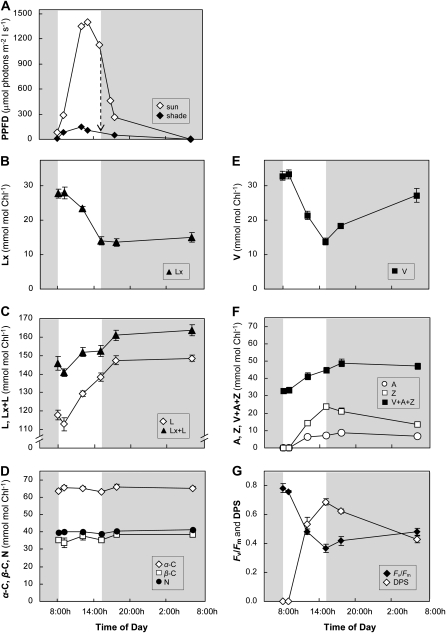
Typical daily photosynthetic photon flux densities (PPFDs) experienced by leaves of avocado plants during growth in the shade enclosure, and on exposure to sun in the open greenhouse, are shown in Figure 1A. The maximum light intensity in the sun (1,400 μmol photons m−2 s−1) was about 10 times higher than the maximum in the shade (150 μmol photons m−2 s−1). Insignificant or no diurnal change in pigment composition and PSII efficiency in leaves that were kept in the shade indicated that neither of the xanthophyll cycles was active in this growth environment (Table III). In fact, neither A nor Z were detectable at any time, and Fv/Fm remained constant at its maximum level. When a shade-grown plant was transferred to sunlight in the unshaded glass house at 8 am (experiment 1), the increase in light intensity from 80 to 290 μmol photons m−2 s−1 by 9 am was insufficient to activate either xanthophyll cycle in mature leaves (Fig. 1, B, C, E, and F). Interestingly, the first apparent effect on the xanthophylls was a decrease in L during this time (Fig. 1C). With further increases in light intensity, xanthophyll deepoxidation was induced, decreasing both Lx and V at similar rates (Fig. 1, B and E). However, the concomitant increase in L and A+Z exceeded the decline in Lx and V up to 2-fold (Fig. 1, B, C, E, and F). Lutein epoxide levels did not recover after the plant was returned to shade, indicating that epoxidation of L in the Lx cycle was either not occurring or was to slow to be detected. Indeed, L continued to increase for another 2 h in the shade, rising to a steady level throughout the following night (Fig. 1, B and C). The Lx+L and V+A+Z pools were each augmented by 15 to 20 mmol mol−1 chlorophyll (Fig. 1, C and F), indicating that substantial de novo xanthophyll synthesis occurred in addition to the deepoxidation reactions.
Figure 1.
Acclimation of mature shade leaves during short-term exposure to sun. The diel PPFDs are shown for the sun and the shade environment in the glasshouse at the canopy level where leaf samples were taken. The dashed arrow indicates the transfer from sun to shade light intensity. Carotenoid pigment profiles and PSII efficiency (Fv/Fm) were determined from a shade-acclimated avocado tree exposed to sun for 7 h and then returned to the shade (gray area). The DPS of the V cycle was calculated as [A+Z]/[V+A+Z]. Values are means ± se (n = 4). Time of day is shown according to the 24-h clock.
Table III.
Steady-state carotenoid pool sizes and PSII efficiency of mature leaves in the shade
The diel responses of shade-acclimated, mature avocado leaves were analyzed in their shade growth environment of the glasshouse. The light intensities are shown in parentheses (PPFD; μmol photons m−2 s−1) and in Figure 1. Carotenoid concentrations are expressed per chlorophyll a+b (mmol mol−1 chlorophyll). A and Z were not detectable (n.d.). Dark-adapted Fv/Fm (arbitrary units) was determined from chlorophyll fluorescence to estimate PSII efficiency. Values are means ± se (n = 4). No statistically significant differences (Tukey multiple comparison test; P > 0.05) were detected between the values in each column.
| Time (PPFD) | N | V | A | Z | Lx | L | α-C | β-C | α-C/β-C | Chlorophyll a/b | Fv/Fm |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 am (10) | 44.3 ± 0.6 | 35.6 ± 0.9 | n.d. | n.d. | 31.6 ± 0.5 | 122.0 ± 2.9 | 62.9 ± 0.6 | 34.7 ± 1.4 | 1.82 ± 0.13 | 2.61 ± 0.06 | 0.79 ± 0.02 |
| 12 pm (150) | 42.2 ± 0.5 | 34.0 ± 0.3 | n.d. | n.d. | 32.9 ± 1.2 | 118.2 ± 2.1 | 64.5 ± 1.0 | 34.6 ± 1.6 | 1.87 ± 0.14 | 2.57 ± 0.06 | 0.78 ± 0.02 |
| 3 pm (80) | 43.9 ± 0.9 | 34.5 ± 0 7 | n.d. | n.d. | 31.5 ± 0 7 | 119.1 ± 3.4 | 62.2 ± 0.7 | 40.4 ± 1.8 | 1.55 ± 0.13 | 2.54 ± 0.15 | 0.77 ± 0.02 |
| 5:30 pm (52) | 45.5 ± 0.3 | 35.4 ± 0.8 | n.d. | n.d. | 32.0 ± 1.3 | 117.6 ± 3.0 | 60.9 ± 1.4 | 39.7 ± 2.0 | 1.54 ± 0.09 | 2.52 ± 0.11 | 0.77 ± 0.02 |
In contrast, epoxidation of A+Z was initiated in the shade without delay. The decrease in A+Z was equal to the overnight increase in V, but 50% of the maximum A+Z levels were still present after one night, reflecting de novo synthesis. However, the respective α-C and β-C pools that are the substrates for L and A+Z synthesis and the N pool that is synthesized from V did not change significantly (Fig. 1D). Overall, the ratio of total change in α-C branch carotenoids and xanthophylls to total change in β-C branch carotenoids and xanthophylls was close to 1 (Table IV). The deepoxidation state (DPS) of the V cycle and PSII efficiency changed in opposite directions. Increased DPS in the sun was accompanied by more than 50% decrease in Fv/Fm, and neither fully recovered during the following 21 h of shade (Fig. 1G). The two parameters were strongly and linearly correlated (r2 = 0.98; data not shown).
Table IV.
Changes in total pool sizes of α-branch compared with β-branch carotenoids during acclimation of sun leaves to shade and shade leaves to sun
The α-branch carotenoid pool [α(C+X)] was calculated as the sum of the means of the α-C+Lx+L pools. The β-branch carotenoid pool [β(C+X)] is the sum of the means of the β-C+V+A+Z+N pools. The differences (Δ) in pool size were assessed by comparing the amounts of pigments at the beginning and end of the respective sun and shade periods. Negative values indicate decreases in total pool size.
| Treatment | Δα(C+X) | Δβ(C+X) | Δα/Δβ(C+X) | |
|---|---|---|---|---|
| mmol mol−1 chlorophyll | ||||
| Shade acclimated → | sun (short term) | |||
| Young (y1)a | 8 h of sun | 36.5 | 41.6 | 0.9 |
| 16 h of shade | 40.0 | 46.2 | 0.9 | |
| Young (y2)a | 8 h of sun | 21.1 | 20.6 | 1.0 |
| 16 h of shade | 44.8 | 46.7 | 1.0 | |
| Matureb | 6–8 h of sun | 10.4 ± 1.8 | 9.1 ± 2.2 | 1.1 |
| 16–18 h of shade | 14.6 ± 1.7 | 13.6 ± 2.2 | 1.1 | |
| Shade acclimated → | sun (long term) | |||
| Youngc | 19 d of sun | 41.2 | 72.6 | 0.6 |
| Maturec | 19 d of sun | 52.2 | 124.0 | 0.4 |
| Sun acclimated → | shade (short term) | |||
| Youngd | 8 h of sun | 9.7 | 8.4 | 1.2 |
| 16 h of shade | 33.5 | 51.0 | 0.7 | |
| Matured | 6–8 h of sun | 19.3 | 14.4 | 1.3 |
| 16 h of shade | 16.3 | 14.8 | 1.1 | |
| Sun acclimated → | shade (long term) | |||
| Younge | 43 d of shade | −35.6 | −31.4 | 1.2 |
| Maturee | 43 d of shade | −36.3 | −35.3 | 1.0 |
Data from experiment 2, first diel.
Data from experiments 1, 4, and 5, first diels (n = 3).
Data from experiment 5, days 0 to 19.
Data from experiment 3, first diel.
Data from experiment 3, days 0 to 43.
Short-Term Sunlight Exposure of Young Shade Leaves Induced Unexpected Synthesis of Lx and L
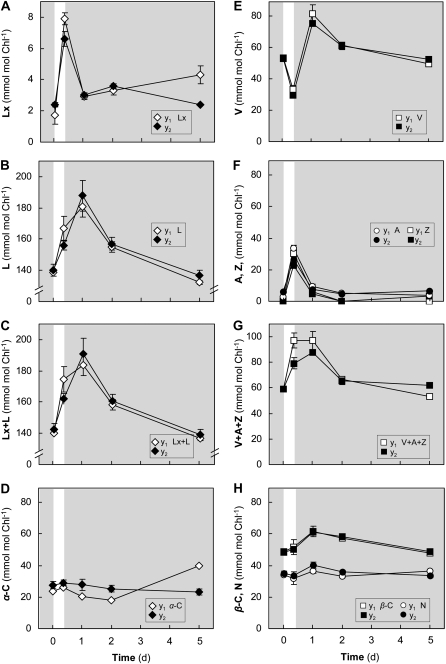
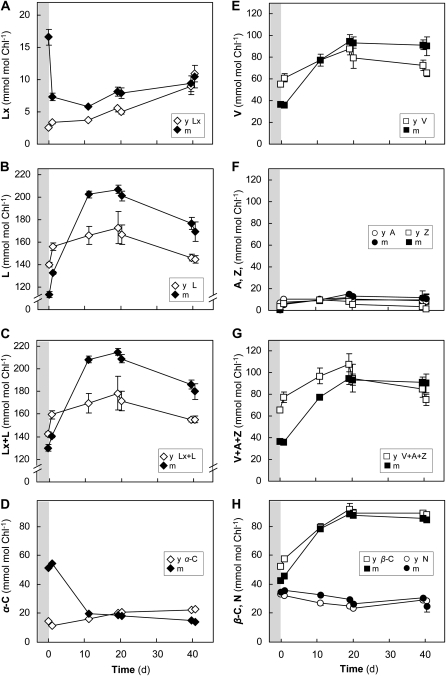
The substantially lower Lx and higher L concentrations in very young avocado leaves that had emerged in the shade (Table I) led us to examine responses of the Lx and V cycle to strong light in these tissues (experiment 2; Fig. 2). In marked contrast to mature leaves, the Lx cycle was initially reversed in young leaves. Levels of Lx increased during sunlight exposure at a rate of 0.6 mmol mol−1 chlorophyll h−1, which was 15 times faster than usually observed in the shade (see below), and Lx actually decreased in the shade overnight, almost returning to initial levels (Fig. 2A). Interestingly, shade-acclimated young leaves contained less than 10% of the Lx found in mature leaves. The L pools increased at a rate of 2 mmol mol−1 chlorophyll h−1 in the first 24 h, both during the sun and the shade, but gradually declined to initial levels over several shade days (Fig. 2B).
Figure 2.
Young shade leaves during short-term exposure to sun (experiment 2). Carotenoid pigments of two sizes of shade-acclimated, young leaves, y1 (white symbols; 6–7 cm in length) and y2 (black symbols; 11–13 cm in length), were compared when exposed to sun for 8 h on day 0 (6-2-6 sampling protocol) and during the following 5 d in the shade indicated by the gray area (6 am samples on days 2 and 5). Values are means ± se (n = 4).
Similar to mature leaves, V decreased in sunlight, and deepoxidation of V accounted for half of the increases in both A and Z (Fig. 2, E and F). Epoxidation of A+Z overnight increased the V pool accordingly, and again a small residual amount of A+Z persisted. The Fv/Fm was linearly and inversely correlated with the V cycle DPS (r2 = 0.79; data not shown).
The increase of the Lx+L pool (45 mmol mol−1 chlorophyll) and the V+A+Z pool (30 mmol mol−1 chlorophyll) by de novo synthesis in young leaves (Fig. 2, C and F) was twice as much as in mature leaves. The young leaves had less than half the amount of α-C than mature leaves but comparable β-C contents. Once again, both carotene pools remained relatively unchanged despite large de novo synthesis of xanthophyll cycle pigments (Fig. 2, D and H), and the equal ratio of α(C+X) to β(C+X) was maintained (Table IV).
Long-Term Shade Acclimation of Mature and Young Sun Leaves Was Dominated by Changes in Lx Cycle Pigments
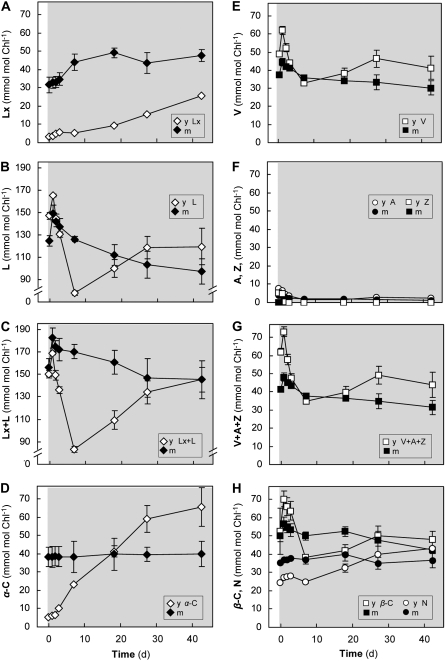
Long-term shade acclimation was examined to discover whether sun leaves could accumulate Lx in the shade and reestablish xanthophyll pigment pools characteristic of shade leaves (experiment 3). A shade-grown plant was acclimated to sun for 30 d and then returned to shade for 43 d (Fig. 3). In spite of the long-term sun exposure, mature and young leaves initially contained amounts of Lx similar to those in shade-grown leaves in experiments 1 and 2. The Lx pools increased in the shade by another 15 to 20 mmol mol−1 chlorophyll in both mature and young leaves (Fig. 3A). However, Lx accumulation (0.041 mmol mol−1 chlorophyll h−1) occurred in mature leaves about 60 times slower than the decrease of Lx by deepoxidation observed during short-term sun exposure (Fig. 1B). In the young sun leaves, Lx accumulation (0.021 mmol mol−1 chlorophyll h−1) was 30 times slower in the shade than in young shade leaves during short-term sun exposure (Fig. 2A). The L pool in mature leaves increased transiently for 1 d after transfer to shade and then decreased by 50 mmol mol−1 chlorophyll (about 2-fold more than the increase in Lx) to stable levels (Fig. 3B). Young leaves showed a similar, transient increase (compare with Fig. 2, B and C), followed by a stronger decline in L pool size (about 90 mmol mol−1 chlorophyll within 7 d), before recovering about 50% of this loss over the next 20 d. In contrast to mature leaves that showed no changes in α-C, the recovery of L in young leaves was accompanied by a quantitatively similar increase in the α-C pool (Fig. 3D).
Figure 3.
Comparison of mature and young sun leaves during long-term acclimation to shade (experiment 3). An avocado tree was exposed to sun for 30 d to establish full sun acclimation of mature (m; black symbols) leaves and to produce new, young sun leaves (y; 11–15 cm in length; white symbols) that expanded to the same size as mature leaves during the experiment. Transfer to shade (gray area) occurred on day 0, and shade acclimation responses of carotenoid pigment pools were examined at intervals (6 am samples) over 43 d. Values are means ± se (n = 4).
V cycle pigment pools generally showed less variation during shade acclimation, and V+A+Z pools were not changed significantly after 43 d (Fig. 3, E–G). The transient increase and decrease of V during the first shade day was more prominent in young leaves and coincided with similar increases in β-C (Fig. 3H). Only traces of A (and no Z) were detectable in both leaf types during the first 4 d, when the V cycle was fully epoxidized (Fig. 3F). The transient increases in V during the first shade day coincided with similar increases in β-C (Fig. 3H), and pools of V or A showed no significant changes after long-term shade acclimation (Fig. 3, E and F). The α(C+X) and β(C+X) pools declined by the same amounts in leaves of both age categories, and the ratio of change was about 1 (Table IV). Thus, long-term shade acclimation of sun leaves primarily affected the pools of Lx cycle pigments in both mature and young leaves and α-C specifically in young leaves. On the whole, the acclimation process seems to be associated with larger fluctuations of pigment pool sizes in the young leaves.
Intermittent Sun Exposure on Mature Leaves during Long-Term Shade Acclimation Induced De Novo Synthesis and Transient Alterations in the Lx and V Cycles
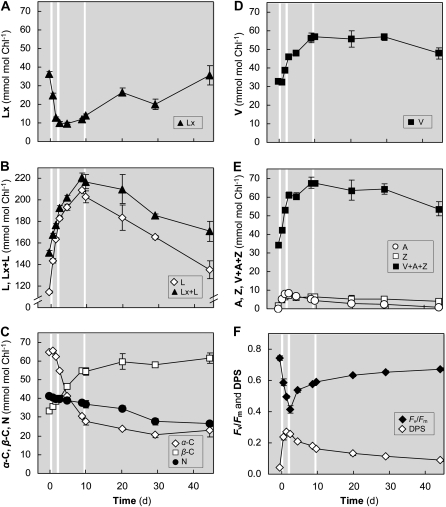
To gain insight into the effect of intermittent sun exposure on long-term shade acclimation, a shade-grown plant was exposed to sunlight from 6 am to 2 pm on days 0, 2, and 9, followed by uninterrupted shade until day 44 (experiment 4). Frequent sampling for pigments and measurements of chlorophyll fluorescence during the first diel (data not shown) confirmed the response pattern of mature shade leaves to short-term sun exposure shown in Figure 1. Changes in pigment composition showed two phases that distinguished days 0 to 10 (intermittent sun exposure) from days 10 to 44 (unperturbed shade). In agreement with experiment 1, sun exposure on days 0 and 2 initiated substantial deepoxidation of Lx and V and de novo pigment synthesis in mature shade leaves, but sun on day 9 had no additive effect (Fig. 4). The increase in L (100 mmol mol−1 chlorophyll), initiated by two separate days of sun, doubled the L pool and was much larger than the decrease in Lx (30 mmol mol−1 chlorophyll) that reduced the Lx pool to 25% of its original size (Fig. 4, A and B). The net increase of the Lx+L pool indicated that de novo synthesis of L was strongly induced in this first phase. During the following unperturbed 35 d in the shade, Lx and L pools readjusted to their initial levels. The 3-fold greater decline in L than increase in Lx during this phase suggested that L epoxidation was accompanied by other turnover processes. Moreover, the extent of truncation of the Lx cycle was apparent from the approximately 130 times faster rate of Lx deepoxidation on day 0 (3.5 mmol mol−1 chlorophyll h−1) compared with L epoxidation from days 10 to 44 (0.027 mmol mol−1 chlorophyll h−1).
Figure 4.
Intermittent sun and shade acclimation responses of mature shade leaves (experiment 4). Shade-acclimated trees were repeatedly exposed to sun (from 6 am to 2 pm on days 0, 2, and 9) and then kept continuously in the shade (gray areas) until day 44. Samples taken at 6 am are shown. Carotenoid pigment pool sizes, DPS of the V cycle, and PSII efficiency (Fv/Fm) are means ± se (n = 4).
Changes in the V cycle pigment pools were less pronounced than in the Lx cycle (Fig. 4, D and E), and although A+Z increased (from 25 to 37 mmol mol−1 chlorophyll) in the sun as expected (2 pm data points not shown), 5 to 15 mmol mol−1 chlorophyll A+Z was detected after each night at 6 am for many days (Fig. 4E). Part of the overnight epoxidation of the A+Z pool during days 0 and 2 contributed to the substantial increase in V (24 mmol mol−1 chlorophyll) that persisted almost throughout the experiment, but the doubling of the V+A+Z pool indicated de novo synthesis of V cycle pigments in addition. The residual A+Z was reflected in sustained DPS (Fig. 4F) and was highly correlated with the Fv/Fm (r2 = 0.88; Fig. 7C) that declined following sun exposure on days 0 to 2 from 0.74 to 0.41 and that recovered to about 80% during long-term shade. As observed previously, there were no significant changes in the α-C or β-C pool sizes during sun exposure on days 0 and 2. However, the decline in α-C (35 mmol mol−1 chlorophyll) over 9 d was matched by an increase in the pool of β-C (Fig. 4C), and the α-C to β-C ratio declined from 1.9 to 0.6.
Figure 7.
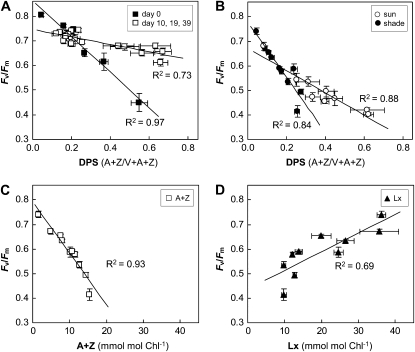
Relationships between PSII efficiency, V cycle deepoxidation, and xanthophyll pigment concentration during sun and shade acclimation. The DPS of the V cycle was calculated as [A+Z]/[V+A+Z], and dark-adapted Fv/Fm was used as an indicator of PSII efficiency. A, Fv/Fm versus DPS in mature and young leaves during long-term sun acclimation in experiment 5 (data from the 6-2-6 protocol; means ± se, n = 4). The slope of the regression line during the first exposure to sun on day 0 (black symbols) differed markedly from those obtained on days 10, 19, and 39 (white symbols). B, Fv/Fm versus DPS in mature leaves during initial sun exposures on days 0 and 2 in experiment 4 (white symbols; means ± se, n = 4) and during long-term shade acclimation (6 am samples; black symbols; means ± se, n = 4). C and D, Fv/Fm versus A+Z (C) and Fv/Fm versus Lx (D) concentrations at 6 am during long-term acclimation to shade after short-term sun exposures in experiment 4.
Long-Term Sun Acclimation of Mature and Young Shade Leaves Showed Large Increases in Both Lx and V Cycle Pigments
Long-term changes in the pigment composition of mature and young shade leaves during sun acclimation were examined in experiment 5 (Figs. 5 and 6). Mature leaves showed the expected 60% deepoxidation of Lx (about 10 mmol mol−1 chlorophyll) when first exposed to sunlight on day 0 (Fig. 5A) but, uncharacteristically, the Lx pool increased slightly from day 10 onward. Young leaves had much lower Lx levels initially (15% of mature leaves), showed the same light stimulation of Lx synthesis and lack of Lx deepoxidation as in the short-term sun exposure (Fig. 2), and accumulated Lx (0.009 mmol mol−1 chlorophyll h−1) to the same level as in mature leaves in the long term (Fig. 5A). The L pool in mature leaves rapidly increased over the first 10 d of sun exposure (Fig. 5B), doubling the pool size, and by day 39 there was still 50% more L than at day 0. The decrease in Lx (10 mmol mol−1 chlorophyll) and α-C (34 mmol mol−1 chlorophyll) during the first 10 d was only half the concurrent increase in L (93 mmol mol−1 chlorophyll; Fig. 5D). Although initially a little higher than in mature leaves, the L pool of young leaves showed larger transients and declined to 25 mmol mol−1 chlorophyll less than in mature leaves after 39 d in the sun. The changes in total Lx+L pools largely matched the changes in the L pools in mature and young leaves (Fig. 5C), showing that de novo synthesis was strongly induced also during long-term sun exposure and that synthesis and turnover were the main processes determining the L pool size. Compared with mature leaves, young shade leaves contained low amounts of α-C (28% of mature leaves) and had more β-C, as observed earlier (Fig. 2). Sun acclimation did not affect α-C in young leaves, but the increase in β-C was similar to that in mature leaves (Fig. 5, D and H).
Figure 5.
Comparison of mature and young shade leaves during long-term acclimation to sun (experiment 5). A shade-acclimated avocado tree was transferred to sun on day 0. Carotenoid pigments were examined in mature (m; black symbols) and young (y; white symbols) leaves at intervals according to the 6-2-6 protocol, and 6 am samples are shown. Values are means ± se (n = 4).
Figure 6.
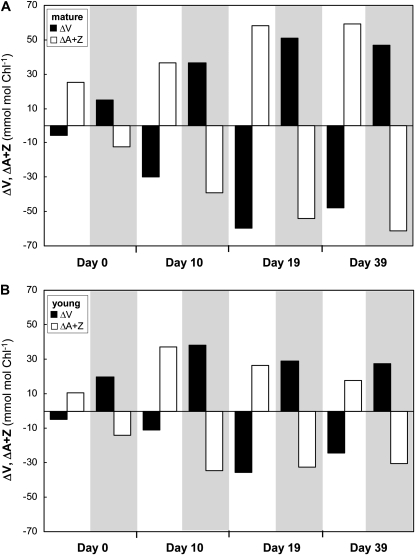
Comparison of diel changes in V cycle xanthophyll pigments of mature (A) and young (B) shade leaves during long-term acclimation to sun (experiment 5). The changes in the amounts of V (ΔV) and A+Z (ΔA+Z) were calculated from the means (n = 4) of the xanthophyll concentrations at the beginning (6 am) and the end (2 pm) of sun exposure (deepoxidation activated) and the beginning (2 pm) and the end (6 am the next morning) of the subsequent shade period (epoxidation activated; gray area). Samples were collected according to the 6-2-6 protocol on days 0, 10, 19, and 39. Leaves were exposed to sun every day throughout the experiment. At each sampling day, the maximum V cycle deepoxidation in the sun period was fully reversed during the following shade period.
The V pool in mature leaves increased much more during the first 18 d (55 mmol mol−1 chlorophyll) than in young leaves over 39 d (10 mmol mol−1 chlorophyll) in the sun (Fig. 5E). Overnight, both leaf types retained similar A and Z pools (up to 26 mmol mol−1 chlorophyll A+Z), so that the V cycle was never fully epoxidized throughout the sun acclimation period (Fig. 5, E and F). De novo synthesis increased the total V+A+Z pool by about the same amount as the β-C pool (Fig. 5, G and H). Interestingly, the increase in α(C+X) during long-term sun acclimation was only about half that of β(C+X) in leaves of both age classes (Table IV).
The functional state of the V cycle was tracked throughout the experiment by comparing the differences in V, A, and Z levels (ΔV, ΔA+Z) at the end of the sun (6 am–2 pm) or the end of the shade (2 pm–6 am) exposure (Fig. 6). Balanced operation of the V cycle (i.e. similar amplitudes of changes in V compared with A+Z) was achieved earlier in mature leaves (after 10 d) than in young leaves (after 19 d). However, the diel changes in the V cycle pigments in younger leaves (Fig. 6B) were also smaller, which most likely reflected differences in the intensity of sun exposure as the blades of young leaves grew in a steeper upward angle in contrast to the nearly horizontal mature leaf blades.
Correlations between PSII Efficiency and Lx and V Cycle Pigments in Avocado Leaves Differed Depending on Age and Light Acclimation States
Correlations between PSII efficiency (Fv/Fm), DPS, and differential Lx and V cycle pigment accumulation of potential physiological relevance were investigated during sun and shade acclimation (Fig. 7). Strong inverse linear correlations were found between Fv/Fm and DPS of the V cycle throughout each diel during long-term sun acclimation (experiment 5), in accordance with the general notion that accumulation of A+Z reduces PSII efficiency. Interestingly, the slope of the regression line was much steeper during the first diel after transfer from shade to sun than during the diels after 10, 19, and 39 d in the sun (Fig. 7A). Likewise, decrease in Fv/Fm correlated well with increased DPS during long-term shade acclimation interrupted by intermittent sun exposure (experiment 4). The slope of declining Fv/Fm against increasing DPS in sunlight during the initial diels (days 0 and 2) was half the slope found between increasing Fv/Fm and decreasing DPS (due to residual A+Z at 6 am) during the slow recovery of PSII efficiency in the following 34 unperturbed shade days (Fig. 7B). In addition to the strong inverse correlation between decline in Fv/Fm and increase in A+Z pool size throughout long-term shade acclimation in this experiment (Fig. 7C), there was a direct, but weaker, correlation between the decline in Fv/Fm and that in Lx (Fig. 7D). Clearly, there was a link between Fv/Fm, DPS, or Lx, but many factors, such as duration of light treatments and/or the age of the leaves, modified these relationships.
DISCUSSION
Loss of Lutein Precedes Engagement of the Lx and V Cycles and De Novo Synthesis of Xanthophylls
Our experiments on shade-to-sun and sun-to-shade acclimation in avocado, a species in which shade leaves accumulate high concentrations of Lx, revealed a variety of novel adjustments in carotenoid pigment pools that may have roles in optimizing photosynthetic efficiency in changing light environments. An interesting observation was that exposure of a shade-grown plant to the natural, gradual increase in irradiance to 1,400 μmol photons m−2 s−1 over 7 h in the sun caused an initial, small decline in L (5–15 mmol mol−1 chlorophyll) prior to measurable deepoxidation of Lx or V (Figs. 1 and 8). A similar but more rapid loss of L over the first 15 to 120 min was also observed when avocado plants were transferred abruptly from shade to full sunlight (Esteban et al., 2008). Evidently, neither deepoxidation of Lx nor de novo synthesis from α-C is sufficiently active to sustain the pool of L in mature shade leaves of avocado under these conditions. One possibility is that this early loss of L is caused by photooxidation before other photoprotection mechanisms are engaged and sufficient Z has accumulated to scavenge singlet oxygen (Havaux and Niyogi, 1999; Johnson et al., 2007). Lutein is a plausible alternative to Z as an effective scavenger of reactive oxygen in vitro (Peng et al., 2006). Although biophysical quenching due to L and Z predominate in the retina of the human eye (Kim et al., 2006), detection of photooxidation products of L and Z (Khachik et al., 1997) indicate that chemical quenching of these substrates also occurs to some extent in this system. Perhaps chemical quenching of L has a role as an early target for photooxidation in the antenna or reaction centers of the photosynthetic apparatus, similar to the D1 reaction center protein of PSII that is most vulnerable to photooxidative damage, thereby protecting the other PSII proteins (Chow et al., 2005). Further evidence is needed to show whether L serves as an antioxidant in response to light stress in vivo.
Figure 8.
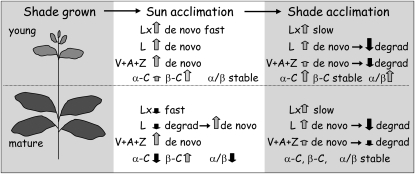
Summary of the distinctive responses of carotenoid pigments during short- and long-term acclimation in avocado leaves. The schematic shoot shows the relative positions and sizes of young and mature leaves in the canopy. Relative changes in pigment pools are represented by the approximate length and direction of the vertical arrows. Horizontal arrows indicate transitions from short- to long-term acclimation. Young leaves stand out due to the reversal of the Lx cycle in the light, with small but rapid synthesis of Lx in the light and decrease in the dark. This is in marked contrast to the rapid deepoxidation of Lx in mature leaves and its very slow recovery of Lx on return to shade. De novo synthesis (de novo) increases total Lx+L and V+A+Z pools during sun acclimation, whereas pigment degradation (degrad) occurs during long-term shade acclimation. The α-C and β-C pools fluctuate in size independent of changes in the derived xanthophylls (Lx+L and V+A+Z, respectively). Young leaves show the fastest and largest absolute changes in α- and β-branch pools, but the ratios of total change in carotenoids and xanthophylls in each branch were close to 1.
Implications for the Concurrent Operation of VDE and ZE in the Lx and V Cycles in Avocado Leaves
Restoration of the Lx pool after fast deepoxidation in just hours of sun exposure is exceptionally slow in mature avocado leaves (Figs. 1, 4, and 8). A month or more in deep shade is required, which is 1 to 2 orders of magnitude slower than in other Lx-accumulating species and represents the slowest recovery of Lx so far reported (García-Plazaola et al., 2007). In contrast, C. reflexa (Bungard et al., 1999) and A. miquelii (Matsubara et al., 2001) essentially complete epoxidation of L overnight, and I. marginata recovers Lx over 5 d in the shade (Matsubara et al., 2008). Species and tissues seem to differ markedly in the extent to which L serves as a substrate for ZE (Bouvier et al., 1996), and depending on the age and light acclimation state of the leaves, epoxidation in avocado may occur 60 to 130 times more slowly than deepoxidation in the Lx cycle. As pointed out earlier, differences in recovery kinetics of V and Lx in various species suggest that ZE has variable affinity for (or access to) L and/or that conditions under which ZE functions in vivo may vary (Matsubara et al., 2003; Snyder et al., 2005). For example, studies in thylakoids of shade leaves of I. sapindoides (Matsubara et al., 2007) led to the hypothesis that photoconverted L was possibly inaccessible to ZE because it had been inserted into the L2 site in antenna Lhcbs.
Other processes may also contribute to the slow recovery of Lx pools in avocado. For example, residual VDE activity in the shade could delay Lx accumulation. However, this seems less likely because VDE requires a low lumen pH of about 5 for association with the antenna, which is a prerequisite for activation (Hieber et al., 2000). One would expect that transthylakoid proton pumping due to photosynthetic electron flow becomes negligible in deep shade and ceases almost immediately in darkness. The transthylakoid ΔpH is dispersed within minutes in Arabidopsis (Arabidopsis thaliana; Ruban et al., 2007), so that the lumen pH should rapidly approach neutral and inactivate VDE. Even though ATP hydrolysis in the dark can sustain lumen acidification and promote VDE activity (Gilmore and Yamamoto, 1992), it seems unlikely to persist for prolonged periods in deep shade. The remarkably truncated Lx cycle in mature leaves of avocado suggests that these tissues may be ideal for testing the roles of the above factors in determining the capacity for Lx synthesis.
Our comparative analysis of mature and young leaves of shade-grown plants opens up even more interesting features of Lx cycle regulation in avocado. Whereas mature leaves showed the typical truncated Lx cycle, the Lx cycle in young leaves was fundamentally different and appeared to function in reverse. During short-term sun exposure, Lx accumulated in the sun and decreased in the shade (Figs. 2 and 8). In fact, this Lx accumulation in the sun was 1 order of magnitude more rapid in young leaves than the slow recovery of Lx pools in mature leaves in the shade. This unusual response is clearly related to the developmental stage of the leaf, since it disappeared between 10 and 20 d as young leaves grew older in sunlight (experiment 5; data not shown). The Lx accumulation in sunlight may be related to the fact that young leaves are sink rather than source leaves (Schaffer et al., 1991) and are likely to have high internal CO2 concentrations that would saturate photosynthetic capacity during leaf expansion and modulate lumen acidification in the chloroplasts at high light intensities. However, we did observe deepoxidation of V in young leaves under these conditions.
Since ZE activity is evidently not confined to very low light and darkness (Frommolt et al., 2001), Lx synthesis in the light may be a “spillover” driven by the very extensive de novo synthesis of L in young leaves. The high ZE activity in young avocado leaves in the light is also suggested by the two times faster epoxidation of A+Z to V. There is little other evidence that the amount of L alone could determine Lx accumulation. The absence of Lx in Arabidopsis lutOE (lutein overexpressor) mutants, in which a significant fraction of V is replaced by L (Pogson and Rissler, 2000), suggests that ZE has low affinity for, access to, and/or activity with L as a substrate in this species. The contrasting responses in the Lx cycle of young versus mature avocado leaves present another challenge for evaluation of ZE and VDE activities in vivo. Priorities for a better understanding of the Lx cycle in avocado include further examinations of pigments in the binding sites of antenna complexes and investigations into the biochemical properties of VDE and ZE in this species.
De Novo Synthesis of Xanthophylls and Flux Regulation through the α- and β-Branches of the Carotenoid Pathways during Photoacclimation
De novo synthesis of L and to a lesser extent of A and Z during sun acclimation may be a distinctive feature of avocado and a few other species (e.g. Anarcardium excelsum; Krause et al., 1999). In contrast, some other tree species show little or no change in L but increased V+A+Z pools when exposed to high light (Logan et al., 1996; Krause et al., 2001), and the low-canopy species Tilia cordata does not adjust L or V+A+Z pools to excess or limiting light in the field, whereas the high-canopy species Populus tremula tends to decrease L in high light and to increase V+A+Z pools (Niinemets et al., 2003). Regulation of carotenoid biosynthesis and degradation is still poorly understood (Cuttriss et al., 2006), and some insights into differential flux rates may be gained from examining relationships between the de novo synthesis of L or A+Z and the pools of their α-C and β-C substrates in our study.
During the first day after transfer to sunlight, most of the increase in L in mature and especially in young leaves can be attributed to de novo synthesis, most of which occurred in the light (Figs. 1 and 2; Table IV). Likewise, additional A and Z were synthesized in young leaves in these experiments, but synthesis of L exceeded that of total V+A+Z by up to 40%. In both cases, carotenoid pools remained unchanged. The flux ratios (de novo synthesis of xanthophyll-pool of carotene substrate) in mature leaves were about 0.5 (Fig. 1), implying that half the α-C and β-C pool turned over in the process. In young leaves, the flux ratio in the α-branch approached 2, compared with a flux ratio of 1 in the β-branch (Fig. 2).
Although flux regulation of de novo xanthophyll synthesis within the α- and β-branches was without impact on carotenoid pools during initial stages of shade-to-sun acclimation, these relationships diverged markedly in the long term. In mature leaves, the L pool nearly doubled in the first 10 d, and about half of this could be attributed to a decrease in α-C. The β-xanthophylls also increased, but in contrast, this was accompanied by a stoichiometric increase of the β-C pool. Successive sun exposures followed by prolonged shade also initiated extensive de novo synthesis of xanthophylls, with relationships to substrate pools similar to those during long-term sun acclimation (compare Figs. 4 and 5). Clearly, long-term regulation of the flux through the α-branch differs markedly from that in the β-branch. These long-term changes in carotenoid pools led to an inversion of the α-C/β-C ratio from about 2 in the shade to 0.5 in the sun (Fig. 8).
Substrate-product relationships in the α- and β-branches of the carotenoid pathway during long-term shade acclimation were especially dynamic in young sun leaves and raise unresolved questions about degradation of L and V. The large transient decline in the L pool size that preceded the increases in both Lx and α-C in young leaves did not occur in mature leaves (Fig. 3). No simple stoichiometric relationships were evident, but the increase in α-C with time in young leaves clearly accounts for the high α-C/β-C characteristic of shade leaves (Thayer and Björkman, 1990).
Evidently, the activities of β,ɛ- and β,β-cyclase, which could determine overall flux from all-trans-lycopene to α-C or β-C, were not differentially regulated during de novo synthesis or degradation of carotenoids of either branch. Changes in total pools of α-C branch and β-C branch pigments were similar in most experiments, with ratios close to 1 (Table IV). Understanding these regulatory processes requires better characterization of biosynthetic enzymes, identification of potential signals, and further knowledge of flux coordination through the α- and β-branches of carotenoid biosynthesis.
Possible Functional Relationships between the Lx and V Cycles and Physiological Implications
There is little doubt that L has essential functions in environments of excess light. It has been proposed that L might augment photoprotection by the β-C pool in Chlamydomonas reinhardtii (Trebst and Depka, 1997) and Arabidopsis (Davison et al., 2002) under high-light stress. Large pools of L may ensure immediate photoprotection in LHCII complexes by serving as a primary biophysical quencher substituting to some extent for the roles ascribed to Z (Ruban et al., 2007). Lutein-deficient mutants of Arabidopsis showed some alterations in Lhcb structure and photosynthetic function (Pogson et al., 1996, 1998; Lokstein et al., 2002; Dall'Osto et al., 2006), and growth and Fv/Fm were reduced under light stress (Kalituho et al., 2007). Also, lack of α- and β-xanthophylls rendered various double mutants in Arabidopsis and C. reinhardtii very susceptible to light stress and impaired growth (Niyogi et al., 1997, 2001).
The best understood function of the V cycle is its relationship to photoprotection and PSII efficiency. The DPS of the V cycle has been viewed as an indicator of NPQ capacity (Demmig-Adams and Adams, 1992), and it is established now that DPS can correlate with both rapidly reversible and sustained NPQ components (Demmig-Adams and Adams, 2006). Our measurements of PSII efficiency and DPS after 30 min of dark adaptation do not reveal the more dynamic energy-dependent (qE) component of NPQ. However, if the efficiency of PSII as measured under our conditions was directly and principally dominated by DPS of the V cycle, Fv/Fm should have shown the same proportional change in response to changes in DPS, independent of the light treatments or age of the leaves throughout these experiments. This was clearly not the case. Even though decreasing Fv/Fm always correlated linearly with increasing DPS in avocado leaves, the magnitude of change in either parameter varied between experiments. The steeper correlation during the first diel on transfer from shade to sun (Fig. 7A) than in subsequent diels as sun acclimation proceeded was not unexpected, because the initially small pools of V cycle pigments presumably offered less photoprotection. Shade leaves presumably also had lower PSII reaction center repair capacity (Chow et al., 2005) during the first diel sun exposure, which could have contributed to stronger photoinactivation and to the much steeper decline in Fv/Fm during the first diel (Fig. 7A). The enlarged V cycle pigment pools as sun acclimation proceeded (Fig. 6) were associated with less decline in Fv/Fm at the same DPS (Fig. 7A), presumably because larger amounts of A+Z confer more effective photoprotection. Additional investigations of changes in fluorescence yields that reflect the operational state of the photosynthetic electron transfer chain in real time during light exposure in relation to DPS are needed to elucidate to what extent and which components of photoprotection/photochemistry and DPS are correlated in varying light environments.
The lower slope of the Fv/Fm versus DPS relationship during the first diel of sun exposure compared with that during the slow recovery of PSII efficiency during 44 d in the shade (Fig. 7B) may be due to the slowly reversible conversion of L to Lx. It has been proposed that photoconverted L “locks in” photoprotective energy dissipation in the shade (Matsubara et al., 2005), which could lead to stronger reduction in Fv/Fm at lower values of DPS. Another possibility is sustained photoinactivation of PSII reaction centers (Matsubara and Chow, 2004) because of limited repair capacity in the shade.
In addition to the putative protective function of photoconverted L in photoprotection, recent studies with Inga species suggested that the accumulation of Lx in the shade in long-lived inner canopy leaves of trees may enhance light-harvesting efficiency. In vitro experiments with thylakoid preparations and reconstitution experiments with recombinant Lhcbs, as well as analysis of fast chlorophyll fluorescence transients in vivo, indicated that less energy is lost by thermal dissipation in the presence of high Lx, possibly facilitating energy transfer between chlorophyll molecules (Matsubara et al., 2007, 2008). The opposing correlations of Lx and A+Z concentration with PSII efficiency in avocado leaves (Fig. 7, C and D) suggest a similar role for Lx in promoting high PSII efficiency in this species. However, our hitherto unreported transitory increase in Lx in young shade leaves transferred to sunlight is counterintuitive to the proposed roles for Lx as an enhancer of light harvesting in deep shade and a repository for L accumulation upon high light exposure (García-Plazaola et al., 2007). Evidently, the relationship between Fv/Fm and DPS is not straightforward in avocado leaves, and it is clear that PSII efficiency, as measured here, is determined by a combination of many factors. Further investigation of these and other possibilities requires detailed kinetic analyses of the decline in Fv/Fm in the light and recovery of PSII efficiency during dark adaptation, as well as of the kinetics of damage to and repair of PSII reaction centers during acclimation.
CONCLUSION
This study revealed an intriguing array of relationships among the pigments of the α- and β-branches of the carotenoid pathways during short-term sun exposure and long-term shade and sun acclimation in shade-grown avocado leaves that are summarized schematically and semiquantitatively in Figure 8. These observations place the relationship of the Lx and V cycles in a new context, suggesting that L may initially confer protection from photooxidation and that subsequent de novo synthesis of L and A+Z may dominate the presumed functional roles of these pigments in the slowly and rapidly reversible down-regulation of light-harvesting efficiency in the antennae of photosystems. It becomes clear that there are significant ontogenetic differences in the light acclimation response, exemplified by the initial sun acclimation response of young leaves associated with reverse Lx cycle activity. In spite of large variations in the α-C and β-C branch carotenoid and xanthophyll pigments, the balance between fluxes through the α-C and β-C branches was equal irrespective of the acclimation processes. Understanding the extent to which these interactions of the two xanthophyll cycles sustain the performance of long-lived avocado leaves in the shade and their contribution to overall canopy photosynthesis, growth, and yield in avocado and other species becomes an exciting direction for future investigation.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Avocado (Persea americana ‘Edranol’) seedlings (60 cm), purchased from Vallance's Nursery in September 2005, were kept in a deeply shaded part of a temperature-controlled (28°C day/18°C night) glasshouse in Canberra. Maximum PPFDs were 50 to 150 μmol photons m−2 s−1 at 12 pm. Seedlings were maintained in their original 8-L containers of potting soil with regular irrigation and additions of slow-release nutrients until preliminary experiments were conducted in November 2005 (García-Plazaola et al., 2007; Esteban et al., 2008). Plants were then pruned to the main stem in April 2006, and the second and third flushes of new leaves were used in experiments during periods of cloudless weather throughout November 2006 and January 2007. Midday light environment in the shade enclosure at the time of the experiments was 30 to 80 and 100 to 150 μmol photons m−2 s−1 for lower canopy leaves and upper canopy leaves, respectively. Maximum sunlight in the unshaded part of the greenhouse was 1,200 to 1,600 μmol photons m−2 s−1 at 12 pm at the upper canopy level.
Light Response and Acclimation Treatments
Plants (1–1.5 m tall) with suitably displayed leaves (i.e. not subject to self-shading) and of requisite age and size were selected so that four similar leaves in each age category were sampled repeatedly by excising leaf discs from the same plant for pigment and fluorescence analysis throughout the experiments. Biological replication was achieved by repeating experiments on comparable leaves on different plants at different times but under similar conditions. Two leaf size categories were used to analyze the influence of age and developmental stage: mature leaves were fully expanded (18–25 cm length), whereas young leaves were rapidly expanding.
Short-term responses (1–5 d) of the Lx and V cycle pigments upon transfer of shade-grown leaves to sun were examined in plants moved to the open glasshouse for exposure to natural sunlight on cloudless days. In experiment 1, the plant was transferred to sun at 8 am and returned to shade at 3 pm after 7 h of exposure to light intensities that increased from 80 to 1,400 μmol photons m−2 s−1. Mature leaves were sampled frequently before, during, and after these transfers and at 6 am the next day. Comparable mature leaves on another plant that remained in the shade enclosure were sampled throughout the day as controls. In experiment 2, young leaves in early (y1; 6–7 cm) and later (y2; 11–13 cm) stages of expansion were sampled before the plant was transferred to the open greenhouse at 6 am (<10 μmol photons m−2 s−1), after 8 h of sun exposure directly before plants were returned to the shade enclosure at 2 pm, and at 6 am the next morning (6-2-6 protocol). In the following shade growth period, samples were taken at 6 am on days 2 and 5. Mature leaves were also sampled as additional controls.
Two types of long-term shade acclimation experiments were performed to examine the truncated nature of the Lx cycle in avocado leaves. In experiment 3, well displayed young (y2, as above) and mature leaves of the current growth flush on a plant that had been acclimated to sunlight for 30 d were analyzed during the 6-2-6 diel on day 0 to establish a baseline for comparison with other experiments and then at 6 am at intervals during the following 43 d in the shade. This experiment simulated the shading of outer canopy leaves that could be expected in rapidly growing shoots of avocado trees in the field. In experiment 4, a shade-grown plant was transferred to sun at 6 am and returned to shade at 2 pm on days 0, 2, and 9. Samples were taken from mature leaves according to the 6-2-6 protocol during these transfers. Subsequently, unperturbed shade acclimation was followed in samples taken at 6 am at intervals until day 44. This experiment may reasonably reflect natural events that temporarily disturb inner canopy light exposures.
Long-term sun acclimation was examined to compare the engagement of the Lx and V cycles in young and mature leaves. This situation may arise when the outer canopy structure is permanently altered by natural factors (e.g. wind damage) or management procedures (e.g. canopy pruning to reduce water use). In experiment 5, a shade-grown plant was transferred to sunlight for 39 d. Leaf samples were collected using the 6-2-6 protocol on days 0, 9, 19, and 38.
Pigment and Chlorophyll Fluorescence Analyses
Leaf discs (1 cm in diameter) were cut out from tip to base of attached leaves for pigment and fluorescence analyses. Chlorophyll fluorescence parameters were determined on leaf discs after 30 min of dark adaptation using the PEA fluorometer (Hansatech) according to the manufacturer's protocol. Maximum efficiency of PSII was derived as Fv/Fm = (Fm − Fo)/Fm (van Kooten and Snel, 1990), where Fm is the maximum dark-adapted fluorescence, Fo is the intrinsic fluorescence in the dark, and Fv is the variable fluorescence. Leaf discs were frozen immediately after fluorescence measurements in liquid nitrogen and stored at −80°C for pigment analysis by HPLC (modified after Pogson et al., 1996). Pigments were extracted in 0.5 mL of acetone to ethyl acetate (60:40, v/v) in a TissueLyser (Qiagen; www.qiagen.com) for 2 min at 30 Hz and then separated into the ethyl acetate phase by addition of 0.4 mL of water and centrifugation at 16,000g at 20°C for 5 min in a microcentrifuge. The pigment-containing ethyl acetate phase was centrifuged again in a new microfuge tube to precipitate any residual particulate matter. Then, 20 μL of the ethyl acetate phase was injected onto a Waters Spherisorb 5 μm ODS2 column for reverse-phase HPLC (Agilent Technologies HP1100 series). Pigments were separated using a linear gradient decreasing solvent A (acetonitrile:water:triethylamine, 90:10:0.1, v/v) from 100% to 33% (v/v) while increasing solvent B (ethyl acetate) from 0% to 67% (v/v) over 31 min, followed by a 4-min elution with 100% (v/v) solvent B at a flow rate of 1 mL min−1. Pigments were identified by retention times and spectra, and concentration on a chlorophyll a+b basis was calculated using peak area conversion factors obtained with pure pigment standards from A440 by Dr. Shizue Matsubara (ICG-III: Phytosphäre, Forschungszentrum Jülich). ANOVA and the Tukey multiple comparison test were applied for statistical analyses.
Acknowledgments
We are grateful to Matthew Gordon for technical support in HPLC measurements, to Steve Dempsey for maintenance of the avocado seedlings, and to Fred Chow for access to chlorophyll fluorescence measuring equipment.
This work was supported by the Australian Research Council (grant nos. DP0666289 to C.B.O. and CE056195 to B.J.P.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Britta Förster (britta.forster@anu.edu.au).
References
- Björkman O (1981) Responses to different quantum flux densities. In OL Lange, PS Nobel, CB Osmond, H Zeigler, eds, Encyclopedia of Plant Physiology: Physiological Plant Ecology. I. Responses to the Physical Environment, Vol 12A. Springer, Berlin, pp 57–107
- Bouvier F, d'Harlingue A, Hugueney P, Marin E, Marion-Poll A, Camara B (1996) Xanthophyll biosynthesis: cloning, expression, functional reconstitution, and regulation of beta-cyclohexenyl carotenoid epoxidase from pepper (Capsicum annuum). J Biol Chem 271 28861–28867 [DOI] [PubMed] [Google Scholar]
- Bungard RA, Ruban AV, Hibberd JM, Press MC, Horton P, Scholes JD (1999) Unusual carotenoid composition and a new type of xanthophyll cycle in plants. Proc Natl Acad Sci USA 96 1135–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow WS, Lee HY, He J, Hendrickson L, Hong YN, Matsubara S (2005) Photoinactivation of photosystem II in leaves. Photosynth Res 84 35–41 [DOI] [PubMed] [Google Scholar]
- Cuttriss AJ, Mimica J, Howitt C, Pogson BJ (2006) Carotenoid metabolism. In R Wise, K Hoober, eds, The Structure and Function of Plastids: Advances in Photosynthesis and Respiration, Vol 23. Springer, Dordrecht, The Netherlands, pp 315–334
- Dall'Osto L, Lico C, Alric J, Giuliano G, Havaux M, Bassi R (2006) Lutein is needed for efficient chlorophyll triplet quenching in the major LHCII antenna complex of higher plants and effective photoprotection in vivo under strong light. BMC Plant Biol 6 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison PA, Hunter CN, Horton P (2002) Overexpression of β-carotene hydroxylase enhances stress tolerance in Arabidopsis. Nature 418 203–206 [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B (1998) Survey of thermal energy dissipation and pigment composition in sun and shade leaves. Plant Cell Physiol 39 474–482 [Google Scholar]
- Demmig-Adams B, Adams WW III (1992) Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol 43 599–626 [Google Scholar]
- Demmig-Adams B, Adams WW III (2006) Photoprotection in an ecological context: the remarkable complexity of thermal energy dissipation. New Phytol 172 11–21 [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams WW III, Mattoo A (2006) Photoprotection, Photoinhibition, Gene Regulation, and Environment: Advances in Photosynthesis and Respiration, Vol 21. Springer, Dordrecht, The Netherlands
- Esteban R, Jiménez ETM, Jiménez S, Morales D, Hormaetxe K, Becerril JM, García-Plazaola JI (2007) Dynamics of violaxanthin and lutein epoxide xanthophyll cycles in Lauraceae tree species under field conditions. Tree Physiol 27 1407–1414 [DOI] [PubMed] [Google Scholar]
- Esteban R, Soledad-Jiménez M, Morales D, Jiménez E, Hormaetxe K, Becerril JM, Osmond C, García-Plazaola JI (2008) Environmental modulation of the lutein epoxide (Lx) cycle in two Lauraceae species. Plant Biol 10 288–297 [DOI] [PubMed] [Google Scholar]
- Förster B, Osmond CB, Pogson BJ (2005) Improved survival of very high light and oxidative stress is conferred by spontaneous gain-of-function mutations in Chlamydomonas. Biochim Biophys Acta 1709 45–57 [DOI] [PubMed] [Google Scholar]
- Frommolt R, Goss R, Wilhelm C (2001) The de-epoxidase and epoxidase reactions of Mantoniella squamata (Prasinophyceae) exhibit different substrate-specific reaction kinetics compared to spinach. Planta 213 446–456 [DOI] [PubMed] [Google Scholar]
- García-Plazaola JI, Hernández A, Olano JM, Becerril JM (2003) The operation of the lutein epoxide cycle correlates with energy dissipation. Funct Plant Biol 30 319–324 [DOI] [PubMed] [Google Scholar]
- García-Plazaola JI, Matsubara S, Osmond CB (2007) The lutein epoxide cycle in higher plants: its relationships to other xanthophyll cycles and possible functions. Funct Plant Biol 34 759–773 [DOI] [PubMed] [Google Scholar]
- Gilmore AM, Yamamoto HY (1992) Dark induction of zeaxanthin-dependent nonphotochemical fluorescence quenching mediated by ATP. Proc Natl Acad Sci USA 89 1899–1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux M, Niyogi KK (1999) The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc Natl Acad Sci USA 96 8762–8767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath R, Arpaia M, Mickelbart M (2005) Avocado tree physiology: understanding the basis of productivity. In Proceedings of the California Avocado Research Symposium. University of California, Riverside, pp 87–119
- Hieber A, Bugos R, Yamamoto H (2000) Plant lipocalins: violaxanthin de-epoxidase and zeaxanthin expoxidase. Biochim Biophys Acta 1482 84–91 [DOI] [PubMed] [Google Scholar]
- Johnson MP, Havaux M, Triantaphylides C, Ksas B, Pascal AA, Robert B, Davison PA, Ruban AV, Horton P (2007) Elevated zeaxanthin bound to oligomeric LHCII enhances the resistance of Arabidopsis to photooxidative stress by a lipid-protective, antioxidant mechanism. J Biol Chem 282 22605–22618 [DOI] [PubMed] [Google Scholar]
- Kalituho L, Rech J, Jahns P (2007) The roles of specific xanthophylls in light utilization. Planta 225 423–439 [DOI] [PubMed] [Google Scholar]
- Khachik F, Bernstein PS, Garland DL (1997) Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Invest Ophthalmol Vis Sci 38 1802–1811 [PubMed] [Google Scholar]
- Kim SR, Nakanishi K, Itagaki Y, Sparrow JR (2006) Photooxidation of A2-PE, a photoreceptor outer segment fluorophore, and protection by lutein and zeaxanthin. Exp Eye Res 82 828–839 [DOI] [PubMed] [Google Scholar]
- Krause GH, Koroleva OY, Dalling JW, Winter K (2001) Acclimation of tropical tree seedlings to excessive light in simulated tree-fall gaps. Plant Cell Environ 24 1345–1352 [Google Scholar]
- Krause GH, Schmude C, Garden H, Koroleva OY, Winter K (1999) Effects of solar ultraviolet radiation on the potential efficiency of photosystem II in leaves of tropical plants. Plant Physiol 121 1349–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latowski D, Grzyb J, Strzałka K (2004) The xanthophyll cycle: molecular mechanism and physiological significance. Acta Physiol Plant 26 197–212 [Google Scholar]
- Liu X, Mickelbart MV, Robinson PW, Hofshi R, Arpaia ML (2002) Photosynthetic characteristics of avocado leaves. Acta Hortic 575 865–874 [Google Scholar]
- Logan BA, Barker DH, Demmig-Adams B, Adams WW III (1996) Acclimation of leaf carotenoid composition and ascorbate levels to gradients in the light environment within an Australian rainforest. Plant Cell Environ 19 1083–1090 [Google Scholar]
- Lokstein H, Tian L, Polle JEW, DellaPenna D (2002) Xanthophyll biosynthetic mutants of Arabidopsis thaliana: altered nonphotochemical quenching of chlorophyll fluorescence is due to changes in photosystem II antenna size and stability. Biochim Biophys Acta 1553 309–319 [DOI] [PubMed] [Google Scholar]
- Matsubara S, Chow WS (2004) Populations of photoinactivated photosystem II reaction centers characterized by chlorophyll a fluorescence lifetime in vivo. Proc Natl Acad Sci USA 101 18234–18239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara S, Gilmore AM, Osmond CB (2001) Diurnal and acclimatory responses of violaxanthin and lutein epoxide in the Australian mistletoe Amyema miquelii. Aust J Plant Physiol 28 793–800 [Google Scholar]
- Matsubara S, Krause GH, Seltmann M, Virgo A, Kursar TA, Jahns P, Winter K (2008) Lutein epoxide cycle, light harvesting and photoprotection in the tropical tree genus Inga. Plant Cell Environ 31 548–561 [DOI] [PubMed] [Google Scholar]
- Matsubara S, Morosinotto T, Bassi R, Christian A-L, Fischer-Schliebs E, Lüttge U, Orthen B, Franco A, Scarano F, Förster B, et al (2003) Occurrence of the lutein-epoxide cycle in mistletoes of the Loranthaceae and Viscaceae. Planta 217 868–879 [DOI] [PubMed] [Google Scholar]
- Matsubara S, Morosinotto T, Osmond B, Bassi R (2007) Short- and long-term operation of the lutein-epoxide cycle in light-harvesting antenna complexes. Plant Physiol 144 926–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara S, Naumann M, Martin R, Nichol C, Rascher U, Morosinotto T, Bassi R, Osmond B (2005) Slowly reversible de-epoxidation of lutein-epoxide in deep shade leaves of a tropical tree legume may ‘lock-in’ lutein-based photoprotection during acclimation to strong light. J Exp Bot 56 461–468 [DOI] [PubMed] [Google Scholar]
- Niinemets U, Kollist H, García-Plazaola JI, Hernández A, Becerril JM (2003) Do the capacity and kinetics for modification of xanthophyll cycle pool size depend on growth irradiance in temperate trees? Plant Cell Environ 26 1787–1801 [Google Scholar]
- Niyogi K, Shih C, Soon Chow W, Pogson B, DellaPenna D, Björkman O (2001) Photoprotection in a zeaxanthin- and lutein-deficient double mutant of Arabidopsis. Photosynth Res 67 139–145 [DOI] [PubMed] [Google Scholar]
- Niyogi KK (2000) Safety valves for photosynthesis. Curr Opin Plant Biol 3 455–460 [DOI] [PubMed] [Google Scholar]
- Niyogi KK, Björkman O, Grossman AR (1997) The roles of specific xanthophylls in photoprotection. Proc Natl Acad Sci USA 94 14162–14167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond CB, Anderson JM, Ball MC, Egerton JJG (1999) Compromising efficiency: the molecular ecology of light resource utilisation in terrestrial plants. In MC Press, JC Scholes, MG Barker, eds, Advances in Physiological Plant Ecology. Blackwell, Oxford, pp 1–24
- Pearcy RW (1990) Sunflecks and photosynthesis in plant canopies. Annu Rev Plant Physiol Plant Mol Biol 41 421–453 [Google Scholar]
- Peng C, Lin Z, Su Y, Lin G, Dou H, Zhao C (2006) The antioxidative function of lutein: electron spin resonance studies and chemical detection. Funct Plant Biol 33 839–846 [DOI] [PubMed] [Google Scholar]
- Pogson BJ, Niyogi KK, Björkman O, DellaPenna D (1998) Altered xanthophyll compositions adversely affect chlorophyll accumulation and nonphotochemical quenching in Arabidopsis mutants. Proc Natl Acad Sci USA 95 13324–13329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogson BJ, Rissler HM (2000) Genetic manipulation of carotenoid biosynthesis and photoprotection. Philos Trans R Soc Lond B Biol Sci 355 1395–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogson BJ, Truong AMK, Britton M, Della G, Penna D (1996) Arabidopsis carotenoid mutants demonstrate that lutein is not essential for photosynthesis in higher plants. Plant Cell 8 1627–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruban AV, Berera R, Ilioaia C, van Stokkum IHM, Kennis JTM, Pascal AA, van Amerongen H, Robert B, Horton P, van Grondelle R (2007) Identification of a mechanism of photoprotective energy dissipation in higher plants. Nature 450 575–578 [DOI] [PubMed] [Google Scholar]
- Schaffer B, Whiley A, Kohli R (1991) Effects of leaf age on gas exchange properties of avocado (Persea americana Mill.). Sci Hortic (Amsterdam) 48 21–28 [Google Scholar]
- Scholefield P, Kriedemann P (1979) Stomatal Development in Avocado Leaves. Research Report (1977–9). CSIRO Division of Horticulture, Adelaide, Australia, pp 50–51
- Snyder AM, Clark BM, Bungard RA (2005) Light-dependent conversion of carotenoids in the parasitic angiosperm Cuscuta reflexa L. Plant Cell Environ 28 1326–1333 [Google Scholar]
- Thayer SS, Björkman O (1990) Leaf xanthophyll content and composition in sun and shade determined by HPLC. Photosynth Res 23 331–343 [DOI] [PubMed] [Google Scholar]
- Trebst A, Depka B (1997) Role of carotene in the rapid turnover and assembly of photosystem II in Chlamydomonas reinhardtii. FEBS Lett 400 359–362 [DOI] [PubMed] [Google Scholar]
- van Kooten O, Snel JFH (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res 25 147–150 [DOI] [PubMed] [Google Scholar]