Abstract
Al3+, the predominant form of solubilized aluminum at pH values below 5.0, has been shown to exert a profound inhibitory effect on root elongation. Al is known to accumulate at the root apex. The plasma membrane represents the first potential target for Al toxicity, due to its pronounced binding to phospholipids. Al appears to alter both the structure and functions of the plasma membrane, and a great deal of research has been conducted concerning the interactions between Al and the plasma membrane. In this review, recent findings regarding the interactions between Al and the plasma membrane are described, specifically findings involving Al-induced alterations in the structure and function of the plasma membrane.
Key Words: acid soil, aluminum, plasma membrane, tolerance, toxicity
Acid Soil
Approximately 70% of soil worldwide is considered to be “problem soil”. These problem soils exhibit a variety of shortcomings or contaminants, including acids, alkali, heavy metals, and high salt concentrations. Acid soils, however, are the most frequently-encountered type of problem soil. Therefore, considerable efforts must be made in order to facilitate the efficient production of food in acid soil. Poor crop growth in acid soils can be directly correlated with the degree of Al saturation in the soil water.1
Al Toxicity in Acid Soils
Under aqueous solutions, Al solubility is known to be highly dependent on pH. At low pH values (pH < 5), the primary species in existence is (Al(H2O)6)3+. Either Al3+, or its hydrated form (Al(H2O)6)3+, is believed to be the principal biologically reactive form.
Al, the most abundant metal within the earth's crust, has been implicated as early as 1918 as a cause of root-growth reduction in barley and rye plants growing in acid soils.2 The root apex is the primary target of Al toxicity. Al exerts effects on a number of different cellular functions, although the foremost effects of Al toxicity remain unclear.3–6 Affected root tips tend to be stubby as the result of these inhibitory effects on cell elongation and cell division.
Association of Al with PM
The PM is the first potential target for Al, due to the high affinity with which Al binds to phosphates. The PM can also function in the manner of a diffusion barrier, protecting the interior of the cell. Therefore, its structure and functions are susceptible to alterations inherent to interactions with metal ions, including Al. In general, this can influence the viscosity of the cell membrane, both in vitro and in vivo. 0.34–8 mM Al3+ has been shown to effect in increase in the microviscosity of rabbit erythrocytes, and 0.01–10 µM Al3+ (pH.5.5) induced an increase in the microviscosity of the erythrocyte membrane, but reduced the microviscosisty of the platelet membrane. The adsorption of Al to liposomes has been evaluated via both equilibrium dialysis and electrophoresis. Al3+ exhibits a 560-fold higher affinity for the phosphatidylcholine surface than that of Ca2+.7 Al3+ at 5 × 10−6 (M) activity is able to neutralize the surface charge of the PM, thereby inducing a shift in surface potential, from −30 to +11 mV. The electron magnetic resonance spectroscopy revealed a dramatic reduction in the membrane fluidity of the microorganism. The lipid phase transition of Thermoplasm acidophilum was observed using AlCl3, at a concentration as low as 10−5 M.8 However, the evidence for the binding of Al to the plant membrane in vivo has yet to be thoroughly investigated. The microsomal fractions of barley roots to which AlCl3 was administered clearly evidenced the binding of Al to phospholipids. By way of contrast, the quantity of Ca2+ associated with the membrane fraction was reduced in this case, thereby indicating that the Ca2+ on the membrane had been replaced by Al.9 Not only phospholipids, but also the proteins in the wheat (Tricum aestivum L.) root PM can be the targets for Al binding.10
The binding of Al to the wheat microsomes and liposomes was determined to proceed in a lipid-dependent manner, with the signal transduction element PIP2 exhibiting the highest degree of affinity for Al, with an Al:lipid stoichiometry of 1:1. Al binding was reduced only in the presence of high Ca2+ (>1 mM) concentrations. Both citrate and, to a lesser extent, malate, proved able to prevent the binding of Al to lipids. This is consistent with the involvement of these organic acids in the detoxification mechanisms inherent to plants.11
Lipid Composition and Al Stress
The PM of the root tips of the Al-tolerant maize cultivar was determined to harbor larger amounts of glucocerebrosides and free sterols than did those of an Al-sensitive maize cultivar. The permeability of nylon capsules coated with PM lipids from the root-tips of the Al-tolerant maize cultivar was found to be lower than that of capsules coated with PM lipids obtained from the Al-sensitive cultivar. Using Naturstoffregenz A (2-aminoethyl diphenyl borate) fluorescent microscopy, larger amounts of flavonoids were determined to have been deposited in the root-tip portion of Al-tolerant signalgrass, as compared what was seen in the other portions and species examined.12 Artificially mixed lipids, which harbored more abundant glucocerebrosides, free sterols, and catechin, exhibited a lower degree of permeability in the Al medium. Therefore, a new feature of Al exclusion, based on this special composition of the PM lipid layer, was proposed.12 Similarly, changes in PM lipids were compared between the Al-tolerant and Al-sensitive wheat genotypes. Zhang et al13 have studied the effects of Al on the lipid composition of the PM from the roots of an Al-resistant (PT741) and an Al-sensitive (Katepwa) cultivar of Triticum aestivum L. Whereas three days of exposure to 20 µM AlCl3 exerted no significant effects on total phospholipids in either genotype, the most abundant phospholipid, namely phosphatidylcholine, was shown to have increased by a significant amount in the Al-resistant PT741, with corresponding reductions in the levels of other phospholipids. By way of comparison, no changes in the phophatidylcholine level were observed in the Al-sensitive Katepwa. Al also reduced the levels of steryl lipids (primarily free sterols) in PT741. No such changes were observed in Katepwa. The ratio of steryl lipids to phospholipids decreased in PT741, whereas no change in this ratio was observed in Katepwa. Al ions bind principally to phospholipids within the membrane, which indicates that the PM may function as a potent barrier to the entry of Al into the cells. Thus, it is possible that a change in the lipid composition of the PM might, conceivably, alter the resistance of the cell, via the exclusion of Al. Delhaize et al14 cloned a wheat cDNA (TaPPS1) which codes for phosphatidylserine synthase (PSS). The wheat TaPPS1 transcript was induced in response to Al stress, and the overexpression of PSS activity resulted in an increase in Al resistance in two yeast strains. However, the high levels of TaPSS1 expression in Arabidopsis and tobacco have been shown to result in the appearance of necrotic lesions on leaves, which may be a result of excessive PS accumulation. Several reports have described Al-mediated interference with membrane lipids, which appears to be induced by the increased production of highly toxic oxygen free radicals. Cakmak and Horst15 determined that lipid peroxidation in the root tips (<2 cm) of the soybean plant could be enhanced by prolonged Al treatment. The Al-dependent increase in lipid peroxidation was amplified by the addition of Fe2+.16 However, Al-associated lipid peroxidation does not appear to constitute the principal cause of the inhibition of root elongation.17
Changes in the Integrity of the PM Induced Alterations in the Function of the PM
The effects of Al on the influx of the calcium, potassium, and ammonium cations, and the nitrate and phosphate anions, were measured in an Al-sensitive barley cultivar (Hordeum vulgare L.). Al (100 µM) was determined to exert an inhibitory effect on the influx of the Ca2+ (69%), NH4+ (40%) and K+ (17%) cations. Al also appeared to interfere with the binding of the cations within the cell wall, by the same order of magnitude as their respective influxes, whereas phosphate binding was shown to be profoundly enhanced. A positive charge layer would retard cation movement and increase the movement of anions to the PM, in proportion to the charge carried by the ions.18
Al (0.37mM) significantly enhanced the permeability of the membrane of the Northern red oak (Quercus rubra) to urea and monoethyl urea, and reduced the permeability of the membrane to water. Al also resulted in a significant alteration in the amount of activation energy required to transport water (+32%), urea (+9%), and monoethyl urea (−7%) across the cell membranes. These changes in membrane behavior may be attributable to an Al-mediated reduction in membrane lipid fluidity and kink frequency, and an Al-mediated augmentation of packing density and an increase in the formation of straight lipid chains.19
The resistance of photosynthesis and growth of the acid-resistant green alga Dunaliella acidophila (optimal growth at pH 1.0) and the salt-resistant Dunaliella parva (grown at pH 7.6) against Al3+, La3+, Cu2+, Cd2+, Hg2+ (applied as chlorides) and W4+ (applied as Na2WO4), as well as the effects of these compounds on the zeta potentials of the cells, were measured in glycerol media of comparable ionic strength. Dunaliella cells with a positive zeta potential (Dunaliella acidophila, pH 1–2) are extremely resistant to toxic di- and trivalent cations, but are quite sensitive to toxic anions. However, cells with a negative zeta potential (D. acidophila and D. parva, pH 7.0) are resistant to toxic anions, but are sensitive to toxic cations. Di- and trivalent cations tend to augment positive zeta potentials, but ameliorate negative zeta potentials. The results suggest that zeta potential plays a pivotal role in cation and anion toxicity.20
Functional Changes Induced by Alterations in PM Potential and Zeta Potential Under Al Stress
The most important functional change occurring in the PM is the Al-mediated induction of membrane potential. Changes in membrane potential appear to be correlated with changes in membrane surface potential (zeta potential), and also appear to regulate ionic flux via a membrane and signal transduction process, which is responsible for the behavior of plants under Al toxicity conditions. Generally speaking, the shifting of PM potential to Al-induced depolarization has been previously observed, but contradictory findings have also been reported. Only two studies have attempted a direct comparison between Al-tolerant and Al-sensitive wheat genotypes. One such study determined that Al hyperpolarized membrane potentials in both genotypes,21 whereas the other study determined that Al elicited a depolarization of the cells in the Al-tolerant, but not the Al-sensitive, cultivars.22
Membrane Potential and Ion Flux
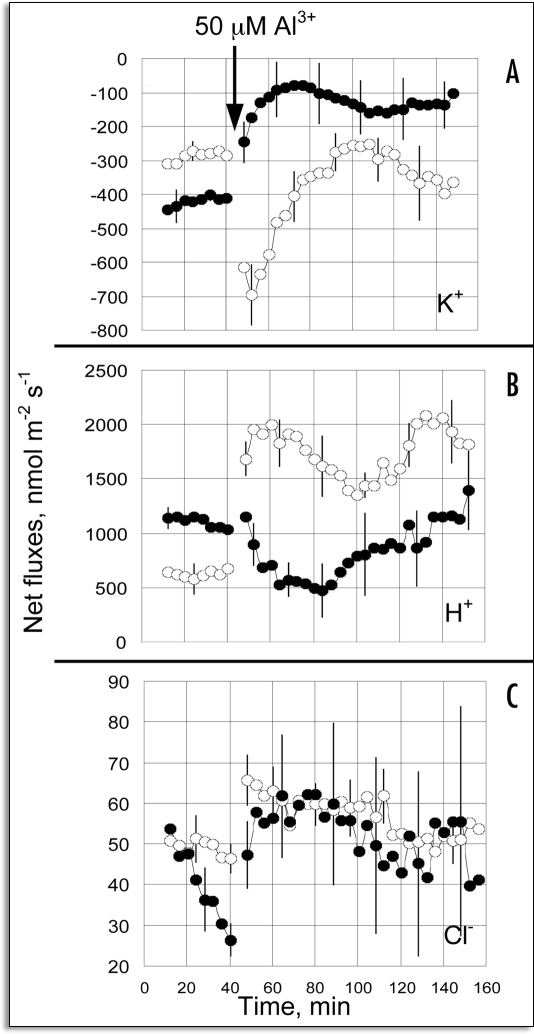
Recently, Wherret et al.23 reported on an interesting trial of the hypothesis that Al-activated ion fluxes might induce changes in the membrane potential (Vm), and that these responses would differ between wheat genotypes that differed with regard to Al tolerance. This study employed two, nearly isogenic lines, an Al-tolerant variant (ET8) and an Al-sensitive variant (ES8). Within minutes of the exposure of the wheat roots to 50 µM AlCl3, a significant degree of depolarization was detected in the Al-treated ET8 variant, but not in the Al-sensitive ES8 variant. They also conducted an investigation into the ion flux that may be responsible for these changes in Vm, via the measurements of real-time fluxes in the concentrations of Cl−, H+ and K+ ions at the root apices of wheat seedlings, using noninvasive microelectrode ion flux estimation (MIFE) methods. The addition of 50 µM AlCl3 to the bathing solution elicited an increase in K+ efflux and H+ influx in the ET8 line, but not in the ES8 line. The differences between the lines persisted for 24 hours, and were observed only in the elongating zone, and not within the meristematic zone. After 24 hours, the Al induced an increase in the Cl− influx in the ET8 line, but inhibited Cl− influx in the ES8 line, in a dose-dependent manner. These results provide new temporal and special information regarding Al-activated ion fluxes in intact wheat plants. The magnitude of the electrical potential difference across a PM (Vm) is a function of the diffusion potential and the effects on the PM exerted by energy-dependent transport systems, including the H+-ATPase system. The movement of ions across a membrane will affect the Vm, unless this movement is exquisitely balanced by the movement of other ions, thereby perpetuating electroneutrality. Al can affect Vm via direct interaction with the membrane, the alteration of membrane zeta potential by the blockage of Ca2+ and K+ channels, or via the inhibition of H+-ATPase. Furthermore, the Al-dependent release of malate anions tends to depolarize until the flow of charge can be balanced by the migrations of other ions. Using this notion as an experimental basis, Wherrett et al23 opted to measure the real time flux in K+, H+, and Cl− under Al stress conditions. No differences in Vm between ET8 and ES8 were detected prior to the addition of Al, that is, −112 ± 2.7 mM at the elongation zone and −156.4 ± 3.9 mV at the mature cells (>10 mm further back). The addition of 50 µM Al to the bathing solution elicited immediate changes in the flux of both K+ and H+ within the elongation zone, but the two genotypes responded differently (Fig. 1). In the ET8 line, Al caused a precipitous increase in K+ efflux, followed by a gradual return and final oscillatory condition at approximate control levels (Fig. 1A). By way of contrast, in the ES8 line, Al induced a rapid and sustained reduction in net K+ efflux within the elongation zone, and this became significantly smaller than was measured in the ET8 line (Fig. 1A). This difference between genotypes was not detected when the fluxes were measured at the root meristems. Furthermore, Al induced a rapid and sustained increase in H+ influx in the elongation zone of the ET8 variant, whereas in the ES8 variant, the influx of H+ was reduced for at least 40 minutes before recovering to control levels (Fig. 1B). Net Cl− fluxes were selectively small, but increases, although minimal, in Cl− influx proved discernible after the addition of Al to both cultivars, again within the elongation zone but not at the meristem (Fig. 1C). After 24 hours of treatment, a reduction was noted in the influx of Cl− both within the elongation zone and at the meristem in the ES8 line, with the influx eventually achieving a negative value. Conversely, Cl− influx increased in a dose-dependent manner in the ET8 line. The magnitude and timing of the Vm changes and ion fluxes are critical to our understanding of the sequence of events by which Al influences membrane potential and ion fluxes, specifically with regard to tolerance and toxicity responses. There is no evidence to suggest the occurrence of a direct interaction between Al and K+ channels, but it can be simply proposed that the stimulation of K+ efflux is triggered by the membrane depolarization induced by the efflux of organic anions. The mechanism by which Al influences channel activity does not appear to depend wholly upon direct binding to channel proteins, but also may be the result of alterations in membrane surface potential. Al exhibits a very high binding affinity for the surfaces of the PM.24,25 This should, then, have a significant impact on those channels whose activity is modified by surface ions or voltage sensitivity, via the regulation of voltage-dependent channels. Al has also been shown to induce significant changes in net H+ fluxes. The ET8 line evidenced a rapid increase in H+-flux, whereas a decrease in H+ flux was observed in the ES8 variant. The enhanced Cl− influx in the ET8 line can account for the stimulation in H+ uptake observed in ET8, as Cl− uptake requires a symport with H+.26
Figure 1.
Transient responses of ion fluxes to Al, measured in the elongation zone. (A) K+ fluxes, (B) H+ fluxes, (C) Cl− fluxes. Data show the responses collected on ET8 plants (open symbols) and ES8 plants (closed symbols) after the addition of 50 µM Al, as shown. Positive values are defined as influxes, and negative values as effluxes. Error bars are s.e.m. (n = 6–8). (From ref. 23).
Zeta Potential and Al Stress
A great deal of attention has been focused on changes in cell membrane surface potential (zeta potential) induced by Al, largely in an effort to elucidate the mechanisms underlying both Al toxicity and Al tolerance. As the zeta potential of the cell membrane is known to regulate the accessibility of Al ions to cells, resulting in functional changes of the membrane, such as ion flux, via H+-ATPase and channel activities. Certain PM properties, including surface negativity, have been demonstrated to be altered by Al, and may prove important as barriers to the passive movement of the Al ion into root cells.27,28 Only a very small amount of information is currently available concerning the relationship between H+-ATPase and zeta potential.29 Since an earlier study by Vose and Randall30 contended that negative surface-charge densities may play pivotal roles in the mechanisms underlying Al tolerance, intensive research in this area has been initiated, starting with investigations in to the cell surface electrical properties of the PM, especially as related to H+ flux under Al stress conditions. A few correlations have been drawn between surface potential and Al tolerance in plants including wheat,27,31,32 maize, and barley.33 However, no study has yet been conducted that integrated both the electrical properties of the PM and alterations in H+-ATPase along various zones of the root apex under Al stress conditions.
Changes in Zeta Potential and PM H+-Atpase Activity
Ahn et al.34–36 recently conducted intensive research regarding changes in zeta potential under Al stress conditions, using squash (Cucuribita pepo L. cv. Tetsukabuto) and two nearly-isogenic wheat lines, differing only in regard to Al tolerance (Al-tolerant ET8 and Al-sensitive ES8). The H+-ATPase activity of the purified PM of squash was reduced substantially (by 20 and 55%) when the roots were exposed for 3 and 6 hours, respectively, to Al. This phenomenon is believed to be associated with transmembrane potential.37 The in vitro effects of Al on H+-ATPase were also assessed. The PM from the control roots was treated with 0, 1, 2, 5, 10, 50 and 100 µM Al in vitro for 10 minutes, and then centrifuged in order to remove any unbound Al. The inhibition of H+-ATPase activity occurred in a dose-dependent manner, and were significant even at 1 µM. 50% inhibition was induced by 10 µM Al. These findings indicated that the H+ flux in the elongation zone of squash is influenced by Al stress. A decrease in PM surface potential (depolarization) was correlated with the decline of H+-ATPase under low temperature38,39 and salt stress29 conditions.
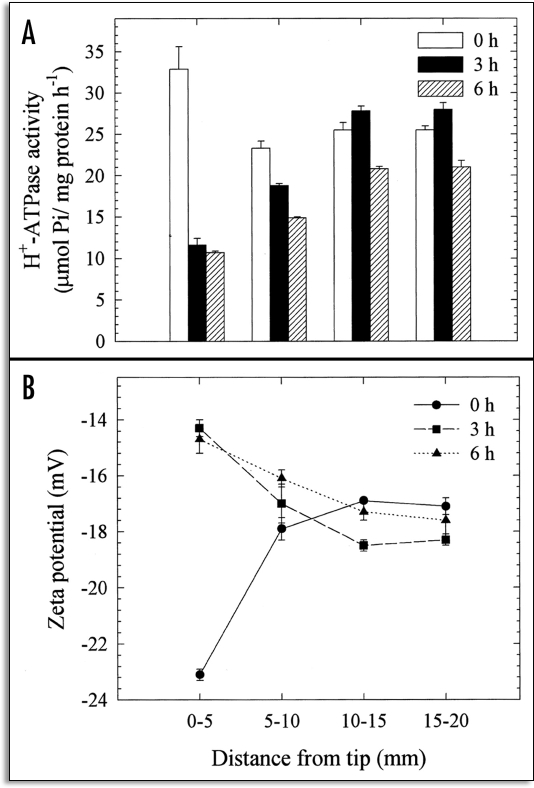
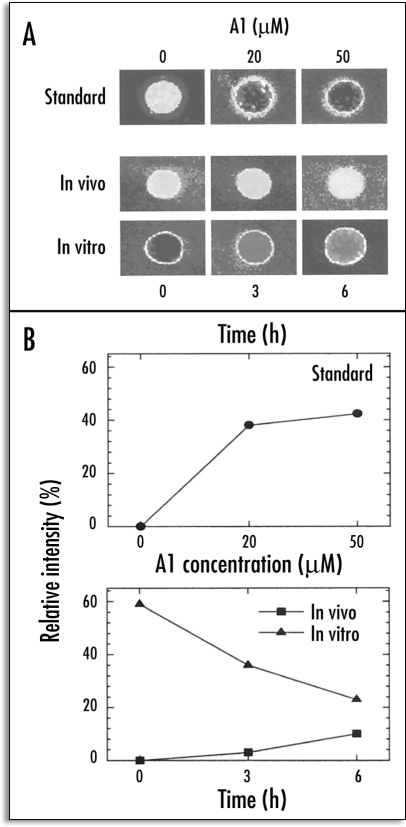
Ahn et al.34 demonstrated the inherent relationship between H+-ATPase activity and the surface potential of the PM, in specific 5 mm segments of squash root. The segmental differences were analyzed with regard to both zeta potential and H+-ATPase activity, as influenced in vivo by Al. As the 2–4 mm region of the root was known to be the most sensitive target site for 50 µM Al, 0–5 mm individual root segments were employed in this trial. The results of segmental analysis showed that the zeta potential was more negative (−22.6 mV) in the 0–5 mm segments than in the other control root segments. A significant increase (from −22.6 to −15 mV) in zeta potential was observed only in the PM vesicles from the 0–5 mm segments after 3 and 6 hours of Al treatment, as compared to the other segments (Fig. 2). In accordance with zeta potential, the H+-ATPase activity of the PM vesicles prepared from the 0–5 mm roots was approximately 30% higher than in the other segments (5–10, 10–15 and 15–20 mm) in the control root, thereby suggesting the developmental control of H+-ATPase activity along the root apex. Intriguingly, the H+-ATPase activity of the PM vesicles isolated from the 0–5 mm root segments treated with Al in vivo were shown to decrease by 64 and 67% after 3 and 6 hours of treatment. These results indicate that the root apex (0–5 mm) is more profoundly sensitive to Al in terms of zeta potential and H+-ATPase activity, due to the higher accumulation of Al therein. The mechanism underlying the inhibition of H+-efflux through the PM as the result of H+-pumping appears to involve the depolarization of surface potential under Al stress conditions. In other words, these observations indicate that more negativity in the resting potential, combined with a higher depolarization of the surface potential, may be associated with sensitivity to Al toxicity. Furthermore, the amount of Al bound to the PM vesicles in vitro was determined after 3 to 6 hours of Al treatment in vivo. The surface potential of the control (0 h Al treatment) PM vesicles was depolarized dramatically after 10 minutes of treatment with 50 µM Al. The binding of Al to the control root (0 h) PM in vitro was noticeably higher, but decreased appreciably with vesicles prepared from roots treated with 50 µM Al in vivo (Fig. 3). At the same time, the further depolarization of PM from roots treated with Al in vivo was apparently reduced. These findings would appear to indicate that the Al bound to PM in vivo could not be removed after the preparation of the vesicles and this tight binding of Al in vivo may have induced an irreversible alteration of the properties of the PM, most notable among which would be the attenuation of Al binding capacity in vitro and an increase in native zeta potential (Fig. 3).
Figure 2.
Effects of Al (50 µM) treatment duration in vivo (0, 3 and 6 h) on the H+-ATPase activity (A) and zeta potential (B) of PM vesicles isolated from specific 5 mm root segment fractions of squash. The plants were grown in Hoagland solution (1/5) adjusted to pH 4.5 for 5 days after germination. Al treatments were conducted in identical solutions, without P, and the plants were cultured in -P solution for at least 12 h prior to treatments. The 5 mm DFTT segments were constructed of approximately 600 individual plants and the isolated PM vesicles were pooled to increase the precision of the measurements. Values are expressed as means ± SE of three replicates, and are representative of two independent experiments. (From ref. 34).
Figure 3.
Visualization of Al-induced alterations of PM properties and Al binding capacity of PM vesicles isolated from squash root apex (0–5 mm) using the Morin assay. (A) Calibration (standard) showing a range of Al concentration and the Al-induced fluorescence of control PM vesicles (top panel); after 50 µM Al treatment in vivo (middle panel); further addition of 50 µM in vitro (10 min) to the same PM vesicles (bottom panel). (B) Quantitative evaluation of the Morin fluorescence based on pixel intensity corresponding to the images presented in (A). Results of standard (top) and after in vivo and in vitro treatments (bottom). (From ref. 34).
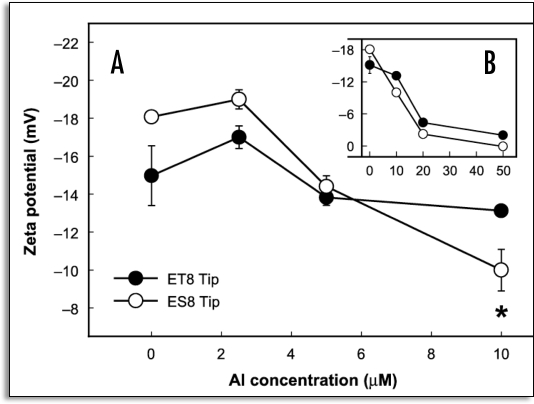
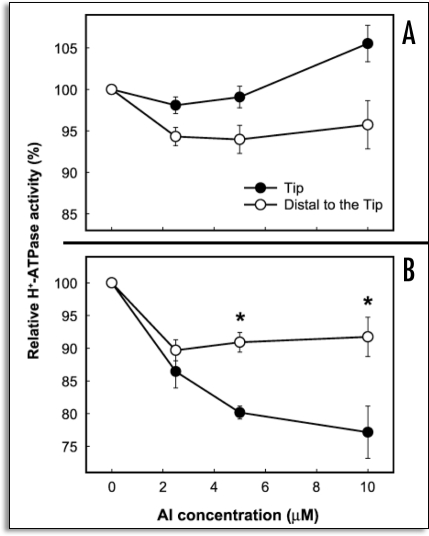
The polyclonal anti-maize PM ATPase antibody was employed to detect H+-ATPase at different root regions in the squash samples treated with Al, as well as in the untreated samples.34 The polyclonal antibody decorated the PM of the whole cells along the root apex of the control. However, the apical zone cells (0–5 mm) apparently exhibited a higher degree of fluorescence as compared to the basal zone cells. The fluorescent intensity of the epidermal cells in the 2–3 mm region decreased after 3 hours of Al treatment, as did that of the cortical cells. However, no obvious differences were detectable in the 7–8 mm mature root zone. By way of contrast, after 6 hours of Al treatment, the apex exhibited a remarkable degree of alteration in fluorescence, as almost no fluorescence could be observed on the PM of the epidermal and cortical cells.34 In accordance with the measured decline in H+-ATPase activity, was also noted a steady decline in H+-ATPase fluorescence in the 2–3 mm region after Al treatment, as compared to all other regions. This may be attributable to an Al-induced reduction in the H+-ATPase protein per unit area of the PM,40 or alterations with regard to the stoichiometric configuration of the autoinhibitory domain within the C-terminal region of H+-ATPase,41 occurring concomitantly with the inhibition of enzyme activity via direct interaction with Al, thereby limiting the cross-reactivity of the antibody with H+-ATPase. Al-induced PM surface potential and H+-ATPase activity in two near-isogenic wheat (Triticum aestivum L.) lines, differing only in terms of Al tolerance, at a single dominant locus (designated Alt1) were utilized. The Al-tolerant (ET8) and Al-sensitive (ES8) wheat lines which are more than 99% identical in terms of genetic background were used. Therefore, it is thought that any differences observed between them are almost certainly associated with the mechanism of Al tolerance due to malate efflux, rather than due to some nonspecific genotypic difference. Recently, the important gene ALMT1 was discovered, and determined to encode for the malate transporter located in the PM of the root tip cells of ET8.42,43 Transgenic barley plants to which ALMT1 had been introduced exuded malate under Al stress conditions, and exhibited Al tolerance.44 We attempted to determine the manner in which the PM surface potential is related to the observed differences in the Al tolerance characteristics of the ET8 and ES8 lines.35 Al treatment was found to elicit a significant depolarization of the zeta potential of PM vesicles isolated from the root tips of ES8 (from −18.5 to −14.3 mV), but hyperpolarized it in the ET8 variant (from −15.1 to −17.9 mV). In the region distal to the root tip, Al was shown to influence the zeta potential of PM only to a slight degree in both wheat lines.35 The treatment of plants for 4 hours with 2.6 µM Al resulted in an increase in H+-ATPase activity in the PM vesicles isolated from the root tips of the ET8 variant (from 26 to 33 µmol Pi mg protein−1), but reduced it in the ES8 line (from 37 to 27 µmol Pi mg protein−1).35 Furthermore, 4 hours of treatment with 2.6 µM Al decreased the H+ transport by 20% in the vesicles of the ES8 root tips, and increased it by 85% in vesicles from the ET8 root tips, as compared to the untreated control samples.35 The mechanism inherent to Al tolerance in wheat is believed to depend on the chelation of toxic Al3+ with the exuded malate, thereby rendering Al less toxic. The deleterious effects of Al on zeta potential and H+-ATPase activity were considerably more profound in the ES8 line than in the ET8 line. The Al-tolerant ET8 excretes 10 times more malate than does the Al-sensitive ES8.3 The question then arises as to whether or not the tolerance of the ET8 line to Al toxicity is induced by (1) decreased Al accumulation as a consequence of malate secretions or (2) via some direct response of the plasma membrane to toxic Al, triggering the observed changes in zeta potential and H+-ATPase activity. Thus, it must be clarified as to whether the effects of Al on surface potential and PM H+-ATPase activity are affected by excreted malate. Experiments were, therefore, conducted under conditions in which malate exudation was avoided or circumvented. The in vitro effects of Al on the zeta potential and H+-ATPase activity of the PM vesicles isolated from ET8 and ES8 were evaluated, in an attempt to ascertain whether or not surface potential and H+-ATPase activity were dependent on inherent differences in the nature of the PMs of ET8 and ES8.35 The PM vesicles were exposed to various Al concentrations (0, 2.5, 5, 10, 20 and 50 µM) for 10 min in vitro. In the controls (0 Al), the zeta potential of the PM vesicles was more negative in the ES8 line than in the ET8 line (Fig. 4). The change in the zeta potential in response to treatment with 10 µM Al was significantly smaller in the ET8 line than in the ES8 line. However, the zeta potential was markedly depolarized by treatment with 20 µM Al in both the ES8 (from −18.5 to −3.0 mV) and ET8 (from −15.5 to −5.0 mV) variants (Fig. 4). The zeta potential was also more negative in the ES8 variant than in the ET8 line at Al concentrations of less than 5 µM, but was less negative at Al concentrations higher than 10 µM. In vitro Al treatment (5 to 10 µM) resulted in a reduction of relative H+-ATPase activity in the PM vesicles isolated from the root tips of the ES8 samples, whereas this activity remained generally unchanged in the ET8 samples (Fig. 5).35 The relative activity of H+-ATPase within the PM vesicles isolated from the region distal to the tip was not influenced by Al treatment in either the ET8 or ES8 lines. PM H+-ATPase appears to play a crucial role in plant survival under a variety of environmental stress conditions. The observed shift of zeta potential of PM to depolarization can be correlated with the decline in H+-ATPase activity observed in PM isolated from squash roots grown under Al toxicity conditions (Fig. 2)34 and in tomato roots grown under salt stress conditions.29 Gimmler et al20 emphasized the importance of the zeta potential in conjunction with metal toxicity in acid-resistant and salt-resistant green algae. From the results observed consequent to Al exposure, the root cell membranes of the ES8 line appear to attract a higher quantity of toxic Al than do the root cell membranes of the ET8 line, due to the greater negative zeta potential of the native PM of the ES8 line, as compared to the ET8 line. In the presence of Al, the rate of depolarization of PM zeta potential in the ES8 line was always higher than in the ET8 at Al concentrations in excess of 10 µM, which inhibits root elongation in ES8 but not in ET8. Therefore the efflux of H+ through PM H+-ATPase might be reduced by the positive surface change of PM in ES8. Furthermore, a lesser quantity of toxic Al was bound to the PM and a large amount of malate anion was exuded in the ET8 line, thereby resulting in a shift of the zeta potential toward the more negative than ES8. This results in the persistence of higher H+-ATPase activity in the ET8 line than in the ES8 line. In the proposed model, the root-cell PM in the Al-sensitive ES8 is depolarized more profoundly than that of ET8, due to more pronounced Al binding and a reduced rate of malate exudation, as well as the fact that H+-ATPase activity is inhibited to a greater extent than in the Al-tolerant ET8, which continues to exude malate, thus maintaining a hyperpolarized plasma membrane (Fig. 6). It appears to be true that the primary mechanism of Al tolerance in ET8 involves the much more robust exudation of malate than is observed with ES8. However, the results of the aforementioned studies revealed that ET8 and ES8 have slightly different PM properties even in the absence of Al ,i.e., ES8 has a more negative inherent membrane surface potential. This indicates that the Alt1 locus, which was identified as differing between the two lines, is potentially pleiotropic. ET8 and ES8 obviously differ with regard to more than just Al-induced malate efflux, thus bringing into question the notion that the Alt1 locus controls only the expression of the PM malate channel. Wherret et al.45 recently suggested that the AKt1 locus may control more than just the PM malate channel in wheat. They also reported that the differential density and regulation of SV channels (ubiquitous slow vacuolar channel) may contribute to the different Ca2+ responses seen in the ET8 and ES8 cultivars, and may also play a role in Al-tolerance.
Figure 4.
Effects of various in vitro Al concentrations, 0, 2.5, 5 and 10 µM (A), and 20 and 50 µM (B) on the zeta potential of PM vesicles isolated from the root tip (apical 10 mm) of ET8 and ES8 grown without Al for 5 days. Five µg of the PM protein (pH 7.4) was treated with Al for 10 min in vitro, and the zeta potential was measured immediately afterwards. The electrophoresis medium was free of Al. The experiment was conducted three times independently, giving similar results. Values are means ± SE (n = 3). Asterisks show statistically different means (*p < 0.05; Student's t-test). (From ref. 35).
Figure 5.
Effects of various Al concentrations (0, 2.5, 5 and 10 µM) in vitro on the relative H+-ATPase activity in PM vesicles isolated from the roots of ET8 (A) and ES8 (B). Seedlings were grown in Al-free 0.2 mM CaCl2 solution, adjusted to a pH of 4.5 for 5 days after germination. The PM vesicles were isolated from the root tips (0–10 mm) and the region distal to the tip (10–20 mm). Five µg of the PM protein (pH 7.4) was then treated with various concentrations of Al for 10 min in vitro, and centrifuged for 1 hour at 100,000 g in order to minimize the carryover of Al from the treatment solution. This experiment was conducted three times, and values from representative experiments are expressed as means ± SE (n = 3). Asterisks indicate statistically different means (*p < 0.05, Student's t-test). (From ref. 35).
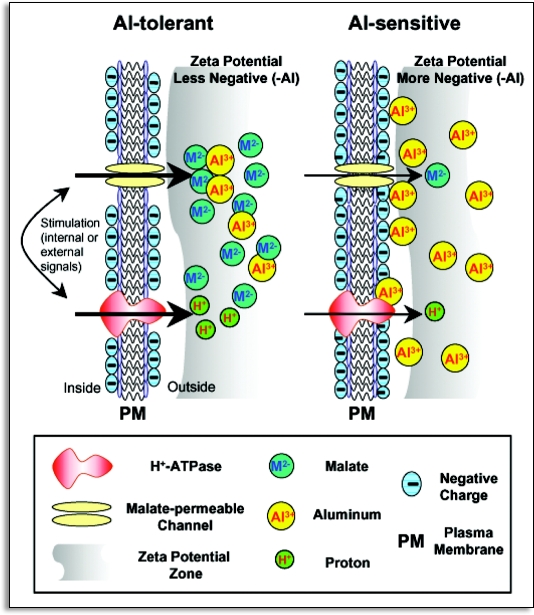
Figure 6.
A schematic diagram illustrating a possible mechanism linking the Al-induced exudation of malate and changes in the properties of PM in Al-tolerant (ET8) and Al-sensitive (ES8) wheat lines. Exudation of malate via malate-permeable channels is accompanied by the hyperpolarization of zeta potential and the enhancement of H+-ATPase activity in Al-tolerant plants. In contrast, in Al-sensitive plants, more negative zeta potential than in Al-tolerant plants attracts more Al3+, causing the depolarization of zeta potential and the decrease in the pumping out of H+ through the PM. The activation of a malate-permeable channel and the H+-ATPase are indicated by arrows of increased thickness in the Al-tolerant plants.
Signal Transduction Affected by Al
For Al, the signal transduction pathway is almost completely undefined, although components of such a pathway have been indicated by some reports. For example, in wheat, Al inhibits a key signal transduction enzyme, designated PLC.28 This suggests that Al may interfere with the phosphoinositide signaling pathway.11 AlCl3 and Al-citrate have been shown to inhibit the PLC of the microsomal membrane, a process which appears to occur in a dose-dependent manner. I50 was observed at Al concentrations of 15–20µM. Exposure to Al exerted no detectable effects on IP3, nor did it affect a range of enzymes isolated from wheat roots, which suggests that Al exposure may specifically target PLC. However, PIP2 is also known to function as a structural anchor for many cytoskeletal components within animal cells. In Dictyostellium, actin polymerization has been experimentally stimulated by IP3.46 Thus Al may exert both direct (via the blockage of IP3-mediated Ca2+ transients) and indirect (via alterations in the cycling of phosphoinositide lipid anchors) effects on cytoskeletal dynamics.11
In wheat, Al also has been shown to transiently induce a protein kinase within 30 seconds of exposure.47 This kinase plays an important role in the secretion of malate by Al. Shen et al.48 reported on the effects of ABA on the efflux of citrate from soybean roots. Al-triggered citrate efflux was found to be sensitive to anion channel inhibitors, including anthracene-9-carboxylic acid(A9C), phenylglyoxal (PG), and niflumic acid (NIF). Pretreatment or treatment with K-252a induced a severe inhibition of Al-induced citrate efflux. Al treatment also affected an increase in endogenous ABA levels in soybean roots, in a dose- and time-dependent manner, whereas K-252a exerted no detectable inhibitory effects on the Al-induced increase in ABA levels. Shen et al.48 reported that ABA is probably involved in early response, after which a K-252a-sensitive protein kinase plays a key regulatory role in the activity of an anion channel within the PM, through which citrate is released from the apical cells of soybean roots. Furthermore, Shen et al.49 conducted an investigation into the regulatory role of PM H+-ATPase on the Al-induced secretion of citrates from soybean roots. Vanadate and fuscicoccin, an inhibitor and activator of PM H+-ATPase, respectively, exerted inhibitory and stimulatory effects on Al-induced citrate secretion. Higher PM H+-ATPase activity was shown to coincide with higher levels of citrate secretion. It was determined that the Al-induced increase in H+-ATPase activity is caused by transcriptional and translatory regulation. Also, PM H+-ATPase activity and expression were determined to be more pronounced in Al-resistant cultivars than in Al-sensitive cultivars. Al activated the threonin-oriented phosphorylation of PM H+-ATPase, in a dose- and time-dependent manner. The upregulation of PM H+-ATPase activity was associated with the secretion of citrate from the roots of the soybean plant.
The effects of Al ions on Ca signaling were assessed in tobacco expressing a Ca2+-monitoring luminescent protein, acquorin, as well as in a newly isolated putative plant Ca2+ channel protein from Arabidopsis thaliana, AtTPC1 (two-pore channel 1). The TPC1 channels were demonstrated to be the only Al-sensitive channel, and were also shown to be responsive to reactive oxygen species and cryptogen, a fungal elicitor protein. Thus, TPC1 channels have been suggested to be involved in Ca signaling, resulting in the development of plant defense mechanisms. The use of Al as a specific inhibitor of TPC1-type plant Ca channels has also been proposed. In a study employing transgenic aequorin-expressing BY-2 tobacco cells, Lin et al.50 presented evidence supporting the involvement of an Al-sensitive signaling pathway requiring TPC1-type channel-dependent Ca2+ influx in the response of plants to salicylic acid, a key plant defense-inducing agent. However, the evidence did not support the involvement of this mechanism in the response of plants to an elicitor prepared from the cell wall of the rice blast disease fungus, Magnaporthe grisea. The involvement of Al-sensitive Ca2+ channels was also evaluated in cold shock response. The data obtained in this trial suggested that the elicitor used had induced Ca2+ influx via an Al-insensitive path, whereas salicylic acid and cold-shock stimulate the influx of Ca2+ via an Al-sensitive pathway.
In order to accurately delineate the signal transduction pathway, it was first necessary to characterize the events occurring within the first minute of Al treatment. Sivaguru et al51 determined that Al depolymerized the microtubles and depolarized the membranes in Arabidopsis, via the activity of calcium channel blockers. They proposed a hypothesis suggesting that Ca2+ influx might involve glutamate receptors, which in animals are ligand-gated cation channels, and are also known to be present in the genome of Arabidopsis. They demonstrated that glutamate depolymerizes the microtubules, and depolarizes the PM. These responses, as well as the inhibition of root elongation, occur within the first few minutes of Al treatment, but are evoked more rapidly by glutamate than by Al. Microtubule depolymerization and membrane depolarization, whether induced by glutamate or by Al, can be blocked by a specific antagonist of ionotropic glutamate receptors, 2-amino-5-phosphonopentanoate, whereas an Al-gated anion channel antagonist blocks the two responses to Al, but not to glutamate. They speculated that Al induces the secretion of glutamate, where it binds to its receptor and triggers the influx of Ca, an influx which results in the observed depolymerization of microtubules and the depolarization of membranes. Consequently, they proposed a probable new glutamate receptor function in plants, as a PM- calcium channel that is mediated by Al. However, regrettably, they neglected to check the possibility that Al binds to glutamate, possibly rendering Al less toxic. The cytoplasmic kinase activity of the PM-associated receptor kinase makes WAKs rather promising candidates for effectors of the Al signal transduction pathway via the utilization of the cell wall-PM-cytoskeleton continuum.52 The Al-induced organ-specific expression of the WAK1 (cell wall-associated receptor kinase 1) gene was therefore investigated, as was the cell type-specific localization of WAK proteins in Arabidopsis. Gene expression in the root fractions evidenced a typical “on” and “off” pattern, thereby suggesting that WAK1 is a further representative of the Al-induced early genes. WAK proteins are localized preferentially to the peripheries of cortical cells, within the elongation zone of the root apex. A lag time was noted to exist between Al-induced WAK transcription and translation. WAKs have been suggested to accumulate at the PM domains, which suffer from mechanical stress and lack a dense array of supporting cortical microtubules. Furthermore, WAK1-overexpressing transgenic plants exhibited an enhanced tolerance for Al with regard to Arabidopsis root growth.53
Microtubules and Al Stress
Some reports have shown that Al depolymerizes microtubules, the result of an increase in cytosolic free Ca2+ induced by the depolarization of PM.54,55 Sivaguru et al.55 indicated that log-phase tobacco cells evidenced no detectable changes in PM potential during the first 30 minutes of Al treatment, but did sustain massive depolarization beginning after 60 minutes of Al treatment. The Al-treated cells manifested a moderate increase in intracellular Ca2+ levels and in the generation of callose, one of the most frequently used Al toxicity markers, after 1 hour. This is consistent with the established time course of PM depolarization. Al toxicity-induced callose generation appears to be dependent on both the depolarization of the PM and on an increase in intracellular Ca2+ levels. Similarly, some studies have shown an increases in the activity of cytoplasmic Ca2+ at the early stages of Al toxicity in the roots of wheat,56 and rye.57
Conclusion
Al3+ solubilized in acidic soil is extremely toxic in terms of root elongation, and is believed to be the primary factor inhibiting plant growth. Therefore, intensive research has been conducted in order to ascertain the mechanisms inherent to Al toxicity and tolerance, on scales from the global to the molecular. Al exhibits a high degree of affinity with phospholipids in the PM, thereby effecting alterations in the structure and functions of the PM. Al exhibits a 560-fold higher affinity for the phosphatidylcholine surface than does Ca2+. Many of the biological activities of PM are altered via the binding of Al, induced primarily via changes in the electrical potential and zeta potential of the PM. The primary Al-induced effects on the PM include the flux of various ions and the signal transduction pathway. Although a great deal of knowledge regarding the effects of Al on the PM has been collected over the past decade, more research will be required regarding the role of the PM in Al-induced stress, in order to advance our understanding of Al stress with regard to the functions of the PM. Future research topics could include:
The identification of specific phospholipids molecule(s) that associate with Al.
Understanding of the effects of Al on PM function, combined with the Al-induced alterations of other ions, particularly Ca2+.
An understanding of the Al-receptor molecules on the PM will be important to our understanding of the process of Al signal transduction in detail.
Acknowledgements
This work was supported in part by Korea Science and Engineering Foundation (KOSEF) to Agricultural Plant Stress Research Center (APARC, R11-2001-09201004-0) of Chonnam National University.
Abbreviations
- PM
plasma membrane
- Al
aluminum
- Vm
membrane potential
- PLC
phospholipase C
- PIP2
phosphoinositol-4,5-biphosphate
- IP3
inositol triphosphate
- ABA
abscisic acid
Footnotes
Previously published onlinse as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/abstract.php?id=2588
References
- 1.Baligar VC, Ahlrichs JL. Nature and distribution of acid soils in the world. In: Schaffert PE, editor. Proceeding of the Workshop to Develop a Strategy for Collaborative Research and Distribution of Technology in Sustainable Crop Production in Acid Savannas and other Problem Soils of the World. Purdue University; 1988. pp. 1–11. [Google Scholar]
- 2.Hertwell B, Pember FR. The presence of aluminum as a reason for the difference in the effect of so-called acid soil on barley and rye. Soil Sci. 1918;6:259–279. [Google Scholar]
- 3.Matsumoto H. Cell biology of Al tolerance and toxicity in higher plants. Int Rev Cytol. 2000;200:1–40. doi: 10.1016/s0074-7696(00)00001-2. [DOI] [PubMed] [Google Scholar]
- 4.Kochia LV. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:237–260. [Google Scholar]
- 5.Haug A, Caldwell CR. Aluminum toxicity in plants: The role of root plasma membrane and calmodulin. In: St John JB, Elliot B, Jacson PC, editors. Frontiers of Membrane Research in Agriculture (Beltsville Symposium 9) Totowa: Rowman and Allanheld; 1985. pp. 359–381. [Google Scholar]
- 6.Kochian LV, Hoekenga OA, Pineros MA. How do crop plants tolerate acid soils? Mechanism of aluminum tolerance and phosphorus efficiency. Annu Rev Plant Physiol Plant Mol Biol. 2004;55:459–493. doi: 10.1146/annurev.arplant.55.031903.141655. [DOI] [PubMed] [Google Scholar]
- 7.Akeson M, Munns DN, Burau RG. Adsorption of Al3+ to phospahtidylcholine vesicles. Biochim Biophys Acta. 1989;984:200–206. doi: 10.1016/0005-2736(89)90269-1. [DOI] [PubMed] [Google Scholar]
- 8.Vierstra R, Haug A. The effect of Al3+ on the physical properties of membrane lipids in Thermoplasma acidophilum+ Biochem Biophys Res Com. 1984;138:138–143. doi: 10.1016/0006-291x(78)90274-7. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto H, Yamamoto, Kasai M. Changes of some properties of the plasma membrane-enriched fraction of barley roots related to aluminum stress: Membrane associated ATPase, aluminum and calcium. Soil Sci Plant Nutr. 1992;38:411–419. [Google Scholar]
- 10.Caldwell CR. Analysis of aluminum and divalent cation binding to wheat root plasma membrane proteins using terbium phosphorescence. Plant Physiol. 1989;91:233–241. doi: 10.1104/pp.91.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones DL, Kochian LV. Aluminum inhibition of the inositol 1,4,5-triphosphate signal transduction pathway in wheat roots: A role in aluminum toxicity? Plant Cell. 1995;7:1913–1922. doi: 10.1105/tpc.7.11.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagatsuma T, Rao IM, Wenzel P, Khan MSH, Tawaraya K, Igarashi K, Murayama T, Kawamura T, Ishikawa S, Uemura M. The plasma membrane plays a key role in high level Al resistance in signalgrass (Brachiaria decumbens): A new feature of Al resistance. In: Li CJ, et al., editors. Plant Nutrition for Food Security Human Health and Environmental Prorection. Tsighua University Press; 2005. pp. 650–651. [Google Scholar]
- 13.Zhang G, Slaski JJ, Archambault DJ, Tayler GJ. Alteration of plasma membrane lipids in aluminum-resistant and aluminum-sensitive wheat genotypes in response to aluminum stress. Physiol Plant. 1997;99:302–308. [Google Scholar]
- 14.Delahaize E, Hebb D, Pichrds KD, Lin JM, Ryan PR, Gardner RC. Cloning and expression of a wheat (Triticum aestivum L.) phosphatidylserine synthase cDNA. J Biol Chem. 1999;274:7082–7088. doi: 10.1074/jbc.274.11.7082. [DOI] [PubMed] [Google Scholar]
- 15.Cakmak I, Horst WJ. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max) Physiol Plant. 1991;83:463–468. [Google Scholar]
- 16.Yamamoto Y, Hachiya A, Matsumoto H. Oxidative damage to membrane by a combination of aluminum and iron in suspension-cultured tobacco cells. Plant Cell Physiol. 1997;38:1333–1339. [Google Scholar]
- 17.Yamamoto Y, Kobayashi Y, Matsumoto H. Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol. 2001;125:199–208. doi: 10.1104/pp.125.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nichol BE, Oliveira LA, Glass ADM, Siddiqi MY. The effects of aluminum on the influx of calcium, potassium, ammonium, nitrate, and phosphate in an aluminum-sensitive cultivar of barley (Hordeum vulgare L.) Plant Physiol. 1993;101:1263–1266. doi: 10.1104/pp.101.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, Sucoff EI, Stadelmann EJ. Aluminum and temperature alteration of cell membrane permeability of Quercus rubra. Plant Physiol. 1991;96:644–649. doi: 10.1104/pp.96.2.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gimmler H, Treffny B, Kowalski M, Zimmermann U. The resistance of Dunaliella acidophila against heavy metals: The importance of the zeta potential. J Plant Physiol. 1991;138:708–716. [Google Scholar]
- 21.Lindberg S, Strid H. Aluminum induces rapid changes in cytoplasmic pH and free calcium and potassium concentrations in root protoplast of wheat (Triticum aestivum) Physiol Plant. 1997;99:405–414. [Google Scholar]
- 22.Papernick LA, Kochian LV. Possible involvement of Al-induced electrical signals in Al tolerance in wheat. Plant Physiol. 1997;115:657–667. doi: 10.1104/pp.115.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wherrett T, Ryan PR, Delhaize E, Shabala S. Effect of aluminum on membrane potential and ion fluxes at the apices of wheat roots. Functional Plant Biol. 2005;32:199–208. doi: 10.1071/FP04210. [DOI] [PubMed] [Google Scholar]
- 24.Suwalsky M, Norris B, Kiss T, Zatta P. Effect of Al(III) speciation on cell membrane and molecular models. Coordination Chemistry Reviews. 2002;228:285–295. [Google Scholar]
- 25.Kinraide TB. Aluminum enhancement of plant growth in acid rooting media. A case of reciprocal alleviation of toxicity by two toxic cations. Physiol Plant. 1993;88:619–625. doi: 10.1111/j.1399-3054.1993.tb01380.x. [DOI] [PubMed] [Google Scholar]
- 26.Sanders D. The mechanism of Cl−1 transport at the plasma membrane of Chara coralline. I. Cotransport with H+ J Memb Biol. 1980;52:129–142. [Google Scholar]
- 27.Kinraide TB, Ryan PR, Kochian LV. Interactive effects of Al3+, H+, and other cations on root elongation considered in terms of cell-surface electrical potential. Plant Physiol. 1992;99:1461–1468. doi: 10.1104/pp.99.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones D, Kochian LV. Aluminum interaction with plasma membrane lipids and enzyme metal binding sites and its potential role in Al cytotoxicity. FEBS Lett. 1997;400:51–57. doi: 10.1016/s0014-5793(96)01319-1. [DOI] [PubMed] [Google Scholar]
- 29.Suhayda CG, Giannini JL, Briskin DP, Shannon MC. Electrostatic changes in Lycopersicon esculentum root plasma membrane resulting from salt stress. Plant Physiol. 1990;93:471–478. doi: 10.1104/pp.93.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vose PB, Randall PJ. Resistance to aluminum and manganese toxicities in plants related to variety and cation-exchange capacity. Nature. 1962;196:85–86. [Google Scholar]
- 31.Kinraide TB, Yermiyahu U, Rytwo G. Computation of surface electrical properties of plant cell membranes. Correspondence to purified zeta potentials from diverse plant sources. Plant Physiol. 1998;118:505–512. doi: 10.1104/pp.118.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kinraide TB. Use of a Gouy-Chapman-Stern model for membrane-surface electrical potential to interpret some features of mineral rhizotoxicity. Plant Physiol. 1994;106:1583–1592. doi: 10.1104/pp.106.4.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagatsuma T, Akiba R. Low surface negativity of root protoplast from aluminum-tolerant plant species. Soil Sci Plant Nutr. 1989;35:443–452. [Google Scholar]
- 34.Ahn SJ, Sivaguru M, Osawa H, Cheng GC, Matsumoto H. Aluminum inhibits the H+-ATPase activity by permanently altering the plasma membrane surface potential in squash roots. Plant Physiol. 2001;126:1381–1390. doi: 10.1104/pp.126.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahn SJ, Rengel Z, Matsumoto H. Aluminum-induced plasma membrane surface potential and H+-ATPase activity in near-isogenic wheat lines differing in tolerance to aluminum. New Phytol. 2004;162:71–79. [Google Scholar]
- 36.Ahn SJ, Sivaguru M, Chung GC, Rengel Z, Matsumoto H. Aluminum-induced growth inhibition is associated with impaired efflux and influx of H+ across the plasma membrane in root apices of squash(Cucurbita repo) J Exp Botany. 2002;53:1959–1966. doi: 10.1093/jxb/erf049. [DOI] [PubMed] [Google Scholar]
- 37.Sasaki M, Kasai M, Yamamoto Y, Matsumoto H. Comparison of the early response to aluminum stress between tolerant and sensitive wheat cultivars; Root growth, aluminum content and efflux of K+ J Plant Nutr. 1994;17:1275–1288. [Google Scholar]
- 38.Ahn SJ, Chung GC, Cho BH. Inducible expression of plasma membrane H+-ATPase in the roots of figleaf gourd plants under chilling root temperature. Physiol Plant. 1999;106:35–40. [Google Scholar]
- 39.Ahn SJ, Chung GC, Seog KY, Cho BH. Sensitivity of plasma membrane H+-ATPase of cucumber root system in response to low root temperature. Plant Cell Reports. 2000;19:831–835. doi: 10.1007/s002999900190. [DOI] [PubMed] [Google Scholar]
- 40.Yan F, Feuerle R, Schaffer S, Fortmeier H, Schubert S. Adaptation of H+-pumping and plasma membrane H+-ATPasze activity in proteoid roots of white lupin under phosphate deficiency. Plant Physiol. 2002;129:50–63. doi: 10.1104/pp.010869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sze H, Li X, Palmgerm MG. Energization of plant cell membrane by H+-pumping ATPase: Regulation and biosynthesis. Plant Cell. 1999;11:677–689. doi: 10.1105/tpc.11.4.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn SJ, Ryan PR, Delhaize E, Matsumoto H. A wheat gene encoding an aluminum activate malate transporter. Plant J. 2004;37:645–657. doi: 10.1111/j.1365-313x.2003.01991.x. [DOI] [PubMed] [Google Scholar]
- 43.Yamaguchi M, Sasaki T, Sivaguru M, Yamamoto Y, Osawa H, Ahn SJ, Matsumoto H. Evidence for the plasma membrane localization of Al-activated malate transporter (ALMT1) Plant Cell Physiol. 2005;46:812–816. doi: 10.1093/pcp/pci083. [DOI] [PubMed] [Google Scholar]
- 44.Delahaize E, Ryan PR, Hebb DM, Yamamoto Y, Sasaki T, Matsumoto H. Enginearing higher level aluminum tolerance in barley with the ALMT1 gene. Proc Natl Acad Sci USA. 2004;101:15249–15254. doi: 10.1073/pnas.0406258101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wherrett T, Shabala S, Pottosin I. Different properties of SV channels in root vacuoles from near isogenic Al-tolerant and Al-sensitive wheat cultivars. FEBS Letters. 2005 doi: 10.1016/j.febslet.2005.11.038. In press. [DOI] [PubMed] [Google Scholar]
- 46.Europe-FGN. Newell K. Inositol 1,4,5-triphospahte and calcium stimulate actin polymerization in Dictyostelium dissideum. J Cell Sci. 1996;82:41–51. doi: 10.1242/jcs.82.1.41. [DOI] [PubMed] [Google Scholar]
- 47.Osawa H, Matsumoto H. Possible involvement of protein phosphorylation in aluminum-responsive malate efflux from wheat root apex. Plant Physiol. 2001;126:411–420. doi: 10.1104/pp.126.1.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen H, Ligaba A, Yamaguchi M, Osawa H, Shibata K, Yan X, Matsumoto H. Effect of K-252a and abscicic acid on the efflux of citrate from soybean roots. J Expt Bot. 2004;55:663–671. doi: 10.1093/jxb/erh058. [DOI] [PubMed] [Google Scholar]
- 49.Shen H, He LF, Sasaki T, Yammoto Y, Zheng SJ, Ligaba A, Yan XL, Ahn SJ, Yamaguchi M, Sasakawa H, Matsumoto H. Citrate secretion coupled with the modulation of soybean root tip under aluminum stress. Upregulation of transcription, translatin, and threonine-oriented phosphorylation of plasma membrane H+-ATPase. Plant Physiol. 2005;138:287–296. doi: 10.1104/pp.104.058065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin C, Yu Y, Kadono T, Iwata M, Umemura K, Furuihi T, Kuse M, Isobe M, Yamamoto Y, Matsumoto H, Yoshizuk K, Kawano T. Action of aluminum, novel TPC1-type channel inhibitor, against salicylate-induced and cold-shock-induced calcium influx in tobacco BY-2 cells. Biochem Biophys Res Commun. 2005;332:823–830. doi: 10.1016/j.bbrc.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 51.Sivaguru M, Pike S, Gassmann W, Baskin TI. Aluminum rapidly depolymerizes cortical microtubles and depolarize the plasma membrane: Evidence that these responses are mediated by a glutamate receptor. Plant Cell Physiol. 2003;44:667–675. doi: 10.1093/pcp/pcg094. [DOI] [PubMed] [Google Scholar]
- 52.Horst J, Schmohl N, Kollemeir M, Baluska F, Sivaguru M. Does aluminum affect root growth on maize through interaction with the cell wall-plasma membrane-cytoskeleton continuum? Plant Soil. 1999;215:163–174. [Google Scholar]
- 53.Sivaguru M, Ezaki B, He ZH, Tong H, Osawa H, Baluska F, Volkmann D, Matsumoto H. Aluminum-induced gene expression and protein localization of a cell wall-associated receptor kinase in Arabidopsis. Plant Physiol. 2003;132:2256–2266. doi: 10.1104/pp.103.022129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sivaguru M, Baluska F, Volkman D, Felle HV, Horst WJ. Impacts of aluminum on the cytoskeleton of the maize root apex. Short-term effects on the distal part of the transition zone. Plant Physiol. 1999;119:1073–1082. doi: 10.1104/pp.119.3.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sivaguru M, Yamamoto Y, Rengel Z, Ahn SJ, Matsumoto H. Early events responsible for aluminum toxicity symptoms in suspension-cultured tobacco cells. New Phytol. 2005;165:99–109. doi: 10.1111/j.1469-8137.2004.01219.x. [DOI] [PubMed] [Google Scholar]
- 56.Zhang WH, Rengel Z. Aluminum induces an increase in cytoplasmic calcium in intact wheat root apical cells. Aust J Plant Physiol. 1999;26:401–409. [Google Scholar]
- 57.Ma QF, Rengel Z, Kuo J. Aluminum toxicity in rye (Secale cereale) root growth and dynamics of cytoplasmic Ca2+ in intact root tips. Ann Bot. 2002;89:241–244. doi: 10.1093/aob/mcf017. [DOI] [PMC free article] [PubMed] [Google Scholar]