Table 3.
Palladium-Catalyzed Carboamination of Substituted N-Protected γ–Aminoalkenes with Functionalized Aryl Bromidesa
| Entry | Amine | Aryl bromide | Product | dr | Rxn. Time (h) |
Yield (%) b |
|---|---|---|---|---|---|---|
| 1 | 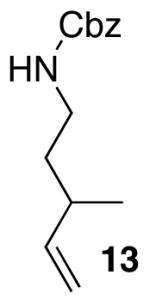 |
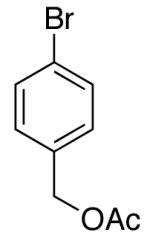 |
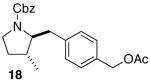 |
12:1 | 20 | 80 |
| 2 | 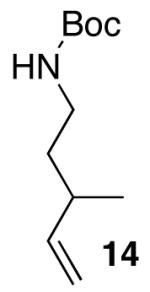 |
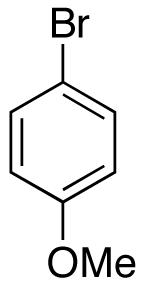 |
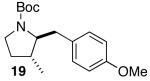 |
15:1 | 16 | 76 |
| 3 | 14 | 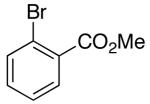 |
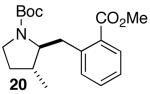 |
14:1 | 18 | 73 |
| 4 | 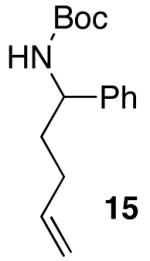 |
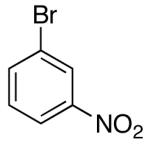 |
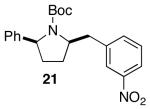 |
>20:1 | 16 | 75 |
| 5 | 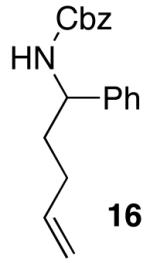 |
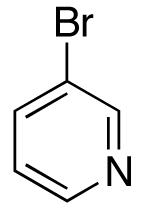 |
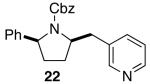 |
>20:1 | 18 | 74 |
| 6 | 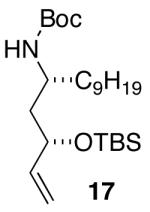 |
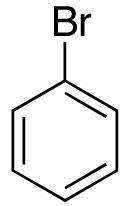 |
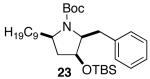 |
>20:1 | 23 18 |
71 (62) c,d |
| 7 | 17 | 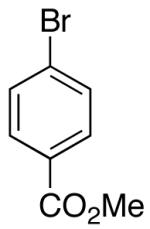 |
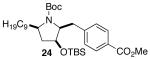 |
>20:1 | 20 | 73 |
Conditions: 1.0 equiv amine, 1.2 equiv ArBr, 2.3 equiv Cs2CO3, 2 mol % Pd(OAc)2, 4 mol % Dpe-phos, dioxane (0.2–0.25 M), 100 °C.
Yield refers to average isolated yield obtained in two or more experiments.
NaO tBu was used in place of Cs2CO3 and toluene was used as solvent.
Pd2(dba)3 was used in place of Pd(OAc)2.
