Table 4.
Carboamination of N-Protected Hex-4-enylaminesa
| Entry | Amine | Aryl bromide |
Product | Rxn. Time (h) |
Yield (%) b |
|---|---|---|---|---|---|
| 1 | 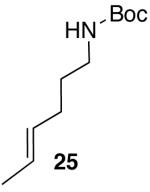 |
 |
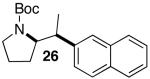 |
23 | 59 |
| 2 | 25 | 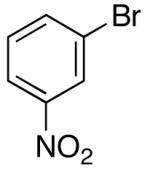 |
 |
36 | 50 |
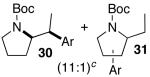
| 3 | 25 | 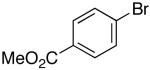 |
 |
44 | 44 e |
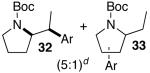
| 4 | 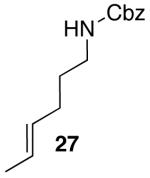 |
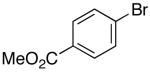 |
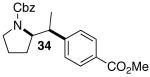 |
42 | 43 e,f |
| 5 | 27 | 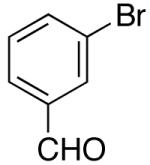 |
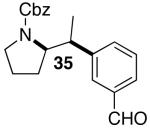 |
30 | 43 e,f, g |
| 6 | 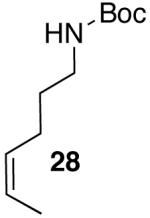 |
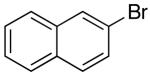 |
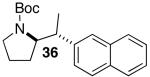 |
27 | 55 e |
| 7 | 28 | 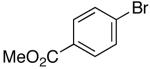 |
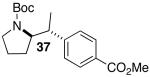 |
21 | 55 e |
| 8 | 28 | 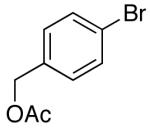 |
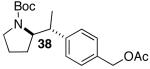 |
31 | 60 e,h |
| 9 | 28 | 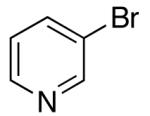 |
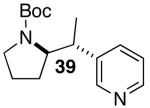 |
53 | 62 e |
| 10 | 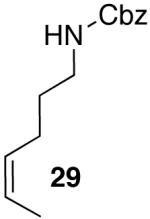 |
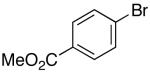 |
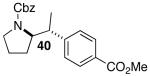 |
53 | 49 e |
| 11 | 29 | 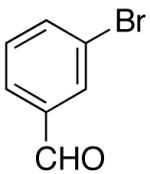 |
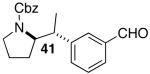 |
53 | 44 e,g |
Conditions: 1.0 equiv amine, 1.2 equiv ArBr, 2.3 equiv Cs2CO3, 5 mol % Pd(OAc)2, 7.5 mol % Nixantphos, dioxane (0.25 M), 100 °C.
Yield refers to average isolated yield obtained in two or more experiments. All reactions proceeded with >20:1 diastereoselectivity.
Ar = 3-nitrophenyl.
Ar = 4-carbomethoxyphenyl.
(±)-BINAP used in place of Nixantphos.
A trace amount (ca. 1–5%) of a regioisomer analogous to 31 and 33 was also obtained.
This reaction was conducted with 2.0 equiv ArBr, 10 mol % Pd(OAc)2 and 15 mol % (±)-BINAP.
This reaction proceeded to 94% conversion.
