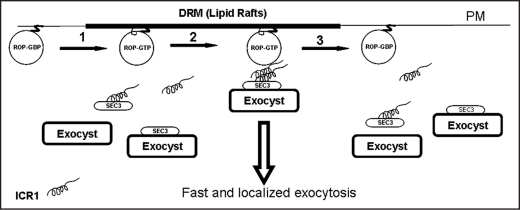
Figure 1.
Possible function of ICR1 and ROPs in localized exocytosis. (1) Activated, GTP-bound, ROPs are transiently acylated on an internal cysteine residue and as a result partition into detergent resistant membranes (DRM) that could be lipid rafts.13 (2) ICR1 monomer or dimer interacts with SEC3 in the cytoplasm and in turn this complex interacts with ROPs at the plasma membrane.23 The ROP-ICR1-SEC3 complex could then serve a landmark for the formation of the exocyst complex. Alternatively, ICR1 may interact with SEC3-exocyst complex or may be a part of the exocyst complex or sub-complex. (3) Upon GTP hydrolysis the ICR1 is released from the ROP and the ICR1-SEC3-exocyst complexes return back to the cytoplasm. The ROP is deacylated and partitions into detergent soluble membranes. The rate of ROP activation/inactivation cycle depends primarily on ROP-GAPs29 and ROP-GEFs and the possible activation/inactivation of the latter by receptor protein kinases in the plasma membrane.30,31 In addition to prenylation and transient acylation, membrane localization of ROPs very likely depends on function of RhoGDIs32 and interaction of the C-terminal polybasic domains of ROPs with phosphatidylphosphoinositides (PIPs).33