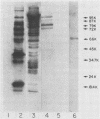
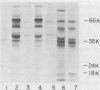
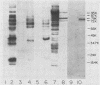
Abstract
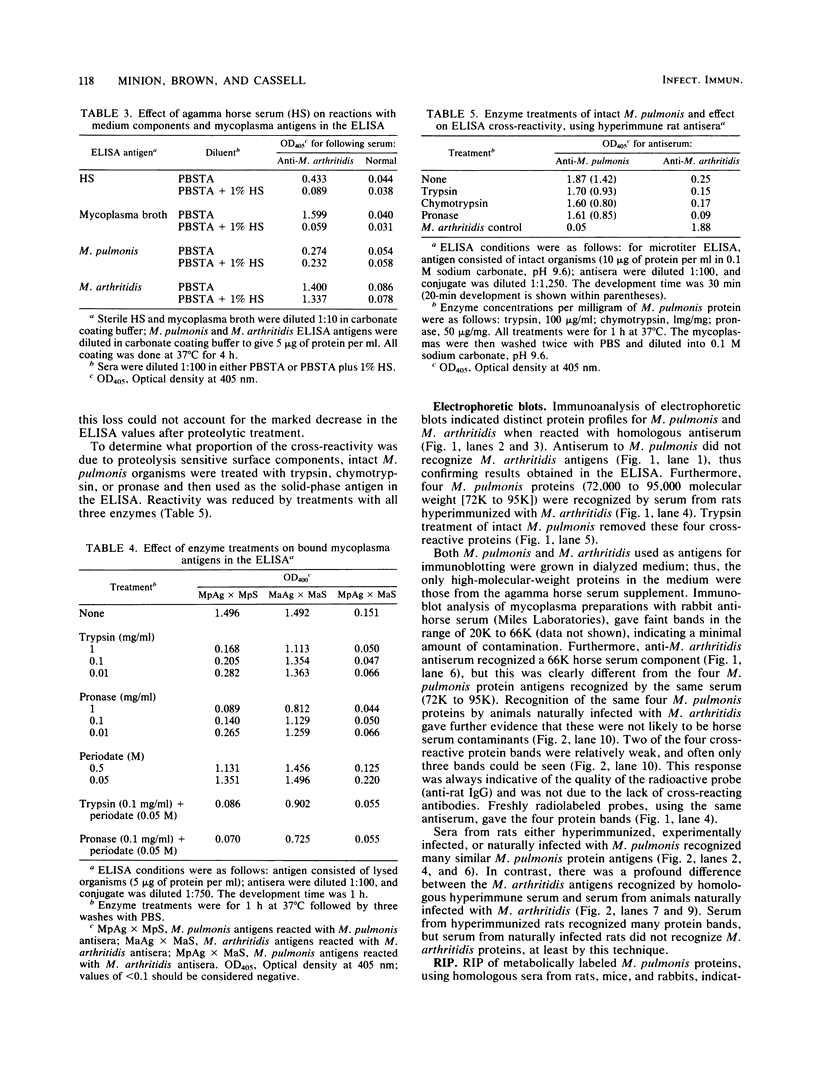
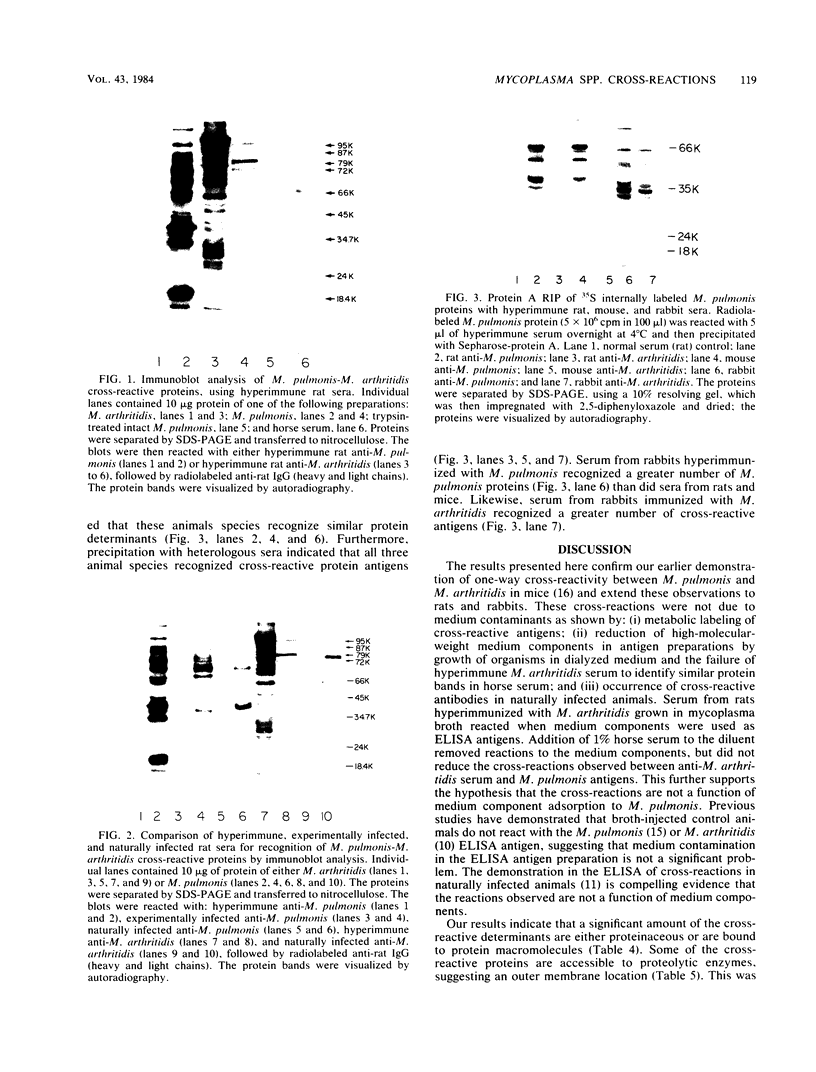
Serological cross-reactivity between Mycoplasma pulmonis and Mycoplasma arthritidis was investigated by enzyme-linked immunosorbent assay, immunoanalysis of electrophoretic blots, and protein A immunoprecipitation reactions. The results demonstrate that one-way cross-reactivity was present in both hyperimmunized and naturally infected rats and that the predominant cross-reactive antigens were M. pulmonis surface proteins. Distinct immunoblot patterns were demonstrated for M. pulmonis and M. arthritidis, allowing differentiation of the two species. The response to M. arthritidis antigens during natural infections differed greatly from that during hyperimmunization. Evidence suggested that nonprotein antigens were major determinants eliciting the antibody response to this mycoplasma.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brown M. B., Cassell G. H., Taylor-Robinson D., Shepard M. C. Measurement of antibody to Ureaplasma urealyticum by an enzyme-linked immunosorbent assay and detection of antibody responses in patients with nongonococcal urethritis. J Clin Microbiol. 1983 Feb;17(2):288–295. doi: 10.1128/jcm.17.2.288-295.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell G. H., Lindsey J. R., Davis J. K., Davidson M. K., Brown M. B., Mayo J. G. Detection of natural Mycoplasma pulmonis infection in rats and mice by an enzyme linked immunosorbent assay (ELISA). Lab Anim Sci. 1981 Dec;31(6):676–682. [PubMed] [Google Scholar]
- Cassell G. H., Lindsey J. R., Davis J. K. Respiratory and genital mycoplasmosis of laboratory rodents: implications for biomedical research. Isr J Med Sci. 1981 Jul;17(7):548–554. [PubMed] [Google Scholar]
- Cassell G. H., Wilborn W. H., Silvers S. H., Minion F. C. Adherence and colonization of Mycoplasma pulmonis to genital epithelium and spermatozoa in rats. Isr J Med Sci. 1981 Jul;17(7):593–598. [PubMed] [Google Scholar]
- Cole B. C., Thorpe R. N., Hassell L. A., Washburn L. R., Ward J. R. Toxicity but not arthritogenicity of Mycoplasma arthritidis for mice associates with the haplotype expressed at the major histocompatibility complex. Infect Immun. 1983 Sep;41(3):1010–1015. doi: 10.1128/iai.41.3.1010-1015.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson L. S., Duthie J. J., Sugar M. Focal Infection in Rheumatoid Arthritis: A Comparison of the Incidence of Foci of Infections in the Upper Respiratory Tract in One Hundred Cases of Rheumatoid Arthritis and One Hundred Controls. Ann Rheum Dis. 1949 Sep;8(3):205–208. doi: 10.1136/ard.8.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson M. K., Lindsey J. R., Brown M. B., Schoeb T. R., Cassell G. H. Comparison of methods for detection of Mycoplasma pulmonis in experimentally and naturally infected rats. J Clin Microbiol. 1981 Dec;14(6):646–655. doi: 10.1128/jcm.14.6.646-655.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- Horowitz S. A., Cassell G. H. Detection of antibodies to Mycoplasma pulmonis by an enzyme-linked immunosorbent assay. Infect Immun. 1978 Oct;22(1):161–170. doi: 10.1128/iai.22.1.161-170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Pollock M. E., Bonner S. V. Comparison of undefined medium and its dialyzable fraction for growth of Mycoplasma. J Bacteriol. 1969 Feb;97(2):522–525. doi: 10.1128/jb.97.2.522-525.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M., Nakagawa M., Kinoshita K., Imaizumi K. Etiological studies on natural outbreaks of pneumonia in mice. Nihon Juigaku Zasshi. 1978 Jun;40(3):283–290. doi: 10.1292/jvms1939.40.283. [DOI] [PubMed] [Google Scholar]
- Sparrow S. The microbiological and parasitological status of laboratory animals from accredited breeders in the United Kingdom. Lab Anim. 1976 Oct;10(10):365–373. doi: 10.1258/002367776780956845. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]