Abstract
Systemic RNA trafficking is emerging as a new paradigm in gene regulation at the whole plant level. The mechanisms that ensure delivery of all sorts of RNAs to their respective cellular destinations remain poorly understood. Several lines of research suggest that specific sequence/structural motif(s) within an RNA directs its trafficking. The first motif was identified in Potato spindle tuber viroid (PSTVd), which mediates trafficking from bundle sheath to mesophyll in young tobacco leaves. Our recent work identified a tertiary structural motif in PSTVd that is required for trafficking from bundle sheath to phloem in Nicotiana benthamiana to initiate long-distance trafficking. This motif consists of a U/C cis Watson-Crick/Watson-Crick base pair with water insertion to widen the minor groove. Similar motifs that are structurally conserved in rRNAs serve as protein-binding sites. These data suggest that distinct RNA motifs interact with specific cellular factors for trafficking across various cellular boundaries to achieve systemic trafficking.
Key words: RNA motif, RNA trafficking, viroid, plasmodesmata, phloem
Intracellular trafficking of proteins, RNAs and other molecules to proper subcellular sites is well established to be essential for the regulation of gene expression and metabolism at the cellular level. An emerging paradigm that impacts studies on gene regulation and metabolism at the organismal level is systemic trafficking of proteins, RNAs and other informational molecules. Knowledge of how an RNA or protein is transported across specific cellular boundaries and eventually into and out of the vascular tissue to reach specific target cells/tissues is crucial for studying mechanisms that regulate developmental and physiological processes at the organismal level. Furthermore, such knowledge is pivotal to understanding viral and viroid infection in a plant. Recent studies on viroid, viral and endogenous RNAs support the concept that RNA has specific motifs to interact with cellular factors to mediate its systemic trafficking across different cellular boundaries.1
Research on viroids provided initial experimental evidence that specific RNA sequence/structural motifs mediate cell-to-cell trafficking. These small, circular RNAs encode no proteins and yet can establish systemic infection in plants.2 Microinjection with in vitro transcripts of Potato spindle tuber viroid (PSTVd) fused to a vector RNA suggested that PSTVd RNA contains a motif to mediate trafficking of the fusion RNA between mesophyll cells in a tobacco leaf.3 The observations that PSTVd RNA is selectively transported into sepals but not the other floral organs in infected Nicotiana benthamiana4 and tomato5 and that two PSTVd strains could enter the phloem for long distance transport but not exit the phloem5 provided further support to the motif hypothesis. Continuous studies on PSTVd led to the first gain-of-function genetic identification of a bipartite motif that is necessary and sufficient to mediate trafficking from bundle sheath to mesophyll, but not in the reverse direction, in young tobacco leaves.6 Importantly, selective and motif-mediated trafficking may also apply to endogenous plant RNAs, even though such motifs remain to be identified.7,8 Furthermore, Lough et al. identified a cis-acting element in the 5′ untranslated region of potexvirus RNA that can mediate cell-to-cell trafficking of a fused green fluorescent protein RNA.9 RNA motif-mediated trafficking may be broadly employed by viral RNAs, as implied by the recent elegant demonstration of Brome mosaic virus RNA trafficking without the participation of viral encoded proteins in N. benthamiana.10
Although previous work provided ample evidence to support the hypothesis that RNA motifs mediate RNA trafficking, some critical issues remained. First, there was no loss-of-function genetic identification of an RNA trafficking motif. Second, the structural nature of a motif is unknown. Third, how an RNA enters the vascular tissue for long-distance trafficking was poorly understood. In our recent work,11 we used PSTVd as a model to identify the first RNA tertiary structural motif required for vascular entry through loss-of-function genetic analyses. This motif comprises nucleotides U43/C318 that forms cis Watson-Crick/Watson-Crick base pairing with water insertion, flanked by short helices comprising nucleotides that interact via canonical cis Watson-Crick/Watson-Crick base pairing (including G:U wobble). The water insertion distorts the local helix by widening the minor groove that facilitates protein or RNA binding. This tertiary structural model was inferred by comparative sequence analysis and comparison with X-ray crystal structures of similar motifs in rRNAs. Extensive mutational analyses showed that all base pair combinations at this location that maintain trafficking function have the distorted local helix, whereas all base pairs that form canonical Watson-Crick/Watson-Crick base pairs (i.e., without water insertion) lost trafficking (and sometimes replication) functions. As further support of this model, several species in the family Pospiviroidae that share similar secondary structures contain identical U/C pair, or a different base pair that is predicted to also distort the local helix, at positions equivalent to U43/C318 of PSTVd. The PSTVd U/C motif likely represents a protein-binding site, based on the known function of the similar motif in rRNAs that interacts with specific proteins. Further detailed analyses of the role of the U/C base pair as well as the surrounding sequences and identification of the protein factor that binds it should further establish this system as a model for mechanistic studies on the molecular regulation of RNA trafficking.
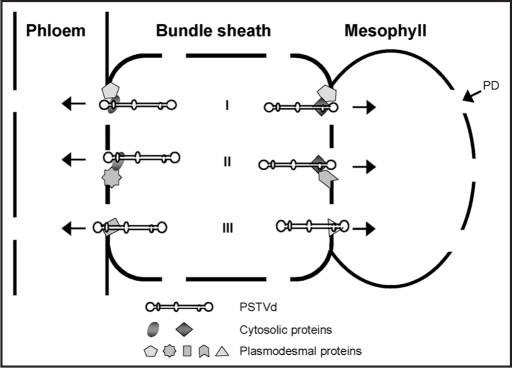
A model emerging from these studies is that an RNA can contain distinct motifs for trafficking across different cellular boundaries. Such motifs may serve as recognition sites by cellular proteins (either cytosolic or plasmodesmal) for trafficking (Fig. 1). Future studies on the identification of viral and cellular RNA trafficking motifs, besides additional viroid trafficking motifs, as well as identification of cellular proteins that interact with motifs should provide a critical test of this model. More importantly, knowledge of the trafficking motifs and their cognate cellular factors will lead to a greater understanding of how RNA trafficking regulates gene expression at the whoel plant level and how infectious RNAs have evolved mechanisms to utilize the endogenous machinery to establish systemic infection.
Figure 1.
Postulated RNA motif-protein interactions that control PSTVd RNA trafficking across different cellular boundaries, in this case from the bundle sheath to phloem and mesophyll, respectively, based on findings reported in references 6 and 11. The specific protein factors remain to be identified. Three scenarios of possible mechanisms are illustrated here but other mechanisms cannot be excluded. Experiments will be needed to test these and alternative mechanisms. In scenario I, different cytosolic proteins that bind distinct RNA motifs interact with the same kind of plasmodesmal protein to traffic PSTVd into the phloem and mesophyll, respectively. In scenario II, different cytosolic proteins that bind distinct RNA motifs interact with different plasmodesmal proteins to traffic PSTVd into the phloem and mesophyll, respectively. In scenario III, RNA motifs interact directly with different plasmodesmal proteins to enable PSTVd movement into the phloem and mesophyll, respectively. PD, plasmodesma.
Acknowledgement
This work was supported by a grant from the US National Science Foundation IOB-0620143.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/4889
References
- 1.Ding B, Itaya A. Control of directional macromolecular trafficking across specific cellular boundaries: A key to integrative plant biology. J Integr Plant Biol. 2007;49:1227–1234. [Google Scholar]
- 2.Ding B, Itaya A. Viroid: A useful model for studying the basic principles of infection and RNA biology. Mol Plant Microbe Interact. 2007;20:7–20. doi: 10.1094/MPMI-20-0007. [DOI] [PubMed] [Google Scholar]
- 3.Ding B, Kwon MO, Hammond R, Owens R. Cell-to-cell movement of potato spindle tuber viroid. Plant J. 1997;12:931–936. doi: 10.1046/j.1365-313x.1997.12040931.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Y, Green L, Woo YM, Owens R, Ding B. Cellular basis of potato spindle tuber viroid systemic movement. Virology. 2001;279:69–77. doi: 10.1006/viro.2000.0724. [DOI] [PubMed] [Google Scholar]
- 5.Zhu Y, Qi Y, Xun Y, Owens R, Ding B. Movement of potato spindle tuber viroid reveals regulatory points of phloem-mediated RNA traffic. Plant Physiol. 2002;130:138–146. doi: 10.1104/pp.006403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qi Y, Pelissier T, Itaya A, Hunt E, Wassenegger M, Ding B. Direct role of a viroid RNA motif in mediating directional RNA trafficking across a specific cellular boundary. Plant Cell. 2004;16:1741–1752. doi: 10.1105/tpc.021980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haywood V, Yu TS, Huang NC, Lucas WJ. Phloem long-distance trafficking of GIBBERELLIC ACID-INSENSITIVE RNA regulates leaf development. Plant J. 2005;42:49–68. doi: 10.1111/j.1365-313X.2005.02351.x. [DOI] [PubMed] [Google Scholar]
- 8.Banerjee AK, Chatterjee M, Yu Y, Suh SG, Miller WA, Hannapel DJ. Dynamics of a Mobile RNA of potato involved in a long-distance signaling pathway. Plant Cell. 2006;18:3443–3457. doi: 10.1105/tpc.106.042473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lough TJ, Lee RH, Emerson SJ, Forster RL, Lucas WJ. Functional analysis of the 5′ untranslated region of potexvirus RNA reveals a role in viral replication and cell-to-cell movement. Virology. 2006;351:455–465. doi: 10.1016/j.virol.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 10.Gopinath K, Kao CC. Replication-independent long-distance trafficking by viral RNAs in Nicotiana benthamiana. Plant Cell. 2007;19:1179–1191. doi: 10.1105/tpc.107.050088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong X, Tao X, Stombaugh J, Leontis N, Ding B. Tertiary structure and function of an RNA motif required for plant vascular entry to initiate systemic trafficking. EMBO J. 2007;26:3836–3846. doi: 10.1038/sj.emboj.7601812. [DOI] [PMC free article] [PubMed] [Google Scholar]