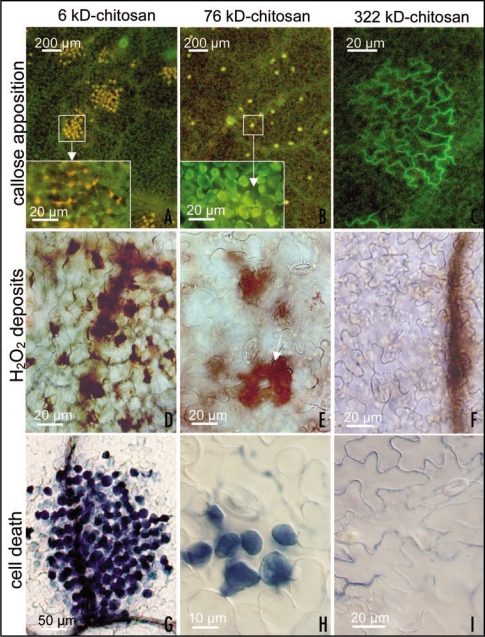
Figure 1.
Different responses induced on Phaseolus vulgaris leaves by treatment with solutions of chitosan with 85% deacetylation degree and different molecular weights; all solutions have been prepared at 0.15% w/v in 0.05 M acetic acid and adjusted at pH 5.6. Callose detection with aniline blue (A–C) 12 h after treatment shows that 76 kD-chitosan elicits the formation of a network of small bright yellow fluorescent spots (B) due to callose apposition between the plasmalemma and the cell wall of some mesophyll cells (see the enlargement in the inset), while 6 kD-chitosan induces lesions involving numerous cells fluorescing in yellow-orange, possibly as consequence of the overlap of phenolics autofluorescence and callose fluorescence (see the enlargement in the inset). In 322 kD chitosan-treated leaves numerous green-fluorescent patches (C), due to chitosan deposits, are present on the leaf epidermis along cell walls, but rarely callose apposition is present. Detection of H2O2 deposits (as brownish precipitates in D–F) with 3-3′-diaminobenzydine (DAB), 24 h after 6 kD-chitosan treatment, indicates that the lesions in (A) are constituted by necrotizing cells as a consequence of extensive H2O2 deposition (D). At the same time, leaves treated with 76 kD-chitosan show moderate H2O2 deposits limited to the same mesophyll cells involved in callose apposition, and often localized around the substomatal cavity into which chitosan can permeate (E, arrow). No H2O2 deposition is present in 322 kD-chitosan treated plants (F). Evans blue staining to detect dead cells (stained in blue) 24 h after treatment (G–I) shows that the lesions in (A) have already evolved in extensive necrotic cell death, while only some of the cells with callose deposition visible in (B) had turned to programmed cell death (H) (as previously shown with other techniques). No dead cells are present in leaves treated with 322 kD-chitosan (I).