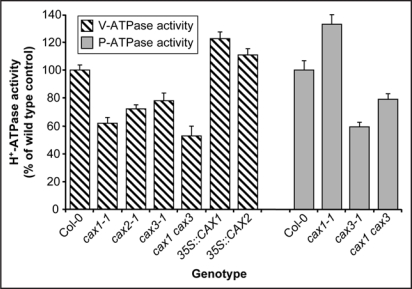
Figure 1.
Tonoplast H+-ATPase (V-ATPase) activity and plasma membrane H+-ATPase (P-ATPase) activity in wild type Arabidopsis (ecotype Col-0) and Arabidopsis lines with manipulated tonoplast Ca2+/H+ exchange activity. 35S::CAX1 and 35S::CAX2 denote lines that overexpress a constitutively active N-terminally truncated CAX1 or CAX2 construct driven by the CaMV 35S promoter in the cax1-1 or cax2-1 mutant background, respectively. V-ATPase H+-transport activity was measured by the ATP-dependent quenching of quinacrine fluorescence, and rates of bafilomycin-sensitive, vanadate-resistant hydrolytic activity of the V-ATPase were determined in isolated tonoplast membranes, as described in refs. 11 and 13. Rates of vanadate-sensitive, bafilomycin- and azide-resistant hydrolytic activity of the P-ATPase were determined in isolated plasma membranes, as described in ref. 14. Results are shown as % of wild type (Col-0) ATPase activity and are means ± SE of 3–4 independent experiments. Data taken and modified from refs. 11–14.