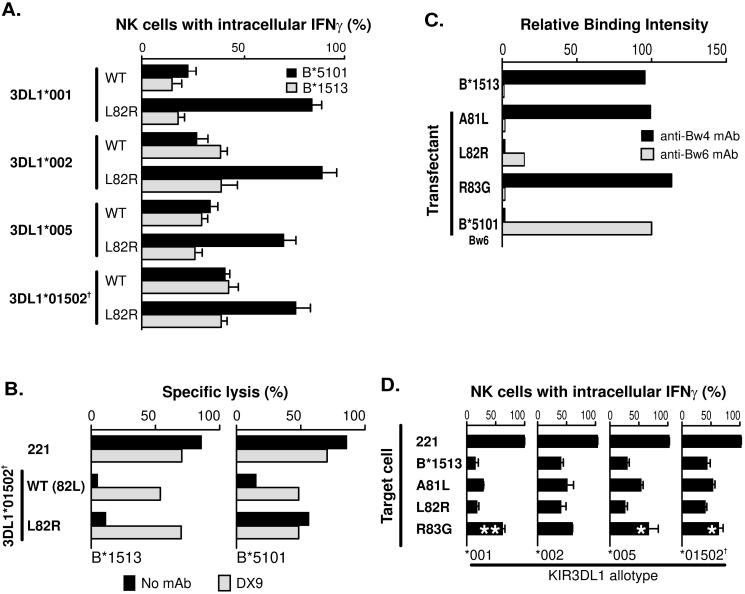
Figure 4. Unlike B*5101, substitution of leucine for arginine at position 82 of B*1513 retains interaction with KIR3DL1.
(A) Compares the interferon-γ response of NK cells expressing defined KIR3DL1 allotypes cultured with 221 cells expressing wild-type (WT) or L82R mutants of HLAB*5101 or -B*1513. (B) Compares the capacity of the B*1513-L82R and B*5101-L82R mutants to engage 3DL1*01502†and inhibit the cytolytic response of NK cells to class I-deficient 221 cells. The experiment was performed three times in duplicate with similar results. Data from a representative experiment is shown in the figure. (C) Compares the binding of anit-Bw4 and anti-Bw6 mAbs to wild type and mutant B*1513. The experiment was performed eight times with similar results. Data from a representative experiment is shown. (D) Shows the capacity of wild-type and mutant B*1513 to bind different KIR3DL1 allotypes and inhibit NK cells. Data shown is the average of three experiments Statistically significant differences between mutant and wild-type are indicated by ***(p < 0.001), ** (p < 0.01), * (p < 0.05) as determined by paired t-test of means. Error bars shown are SEM. Cell surface expression level of B*1513 and its mutants was monitored with W6/32 with all transfectants expressing similar levels [data not shown].