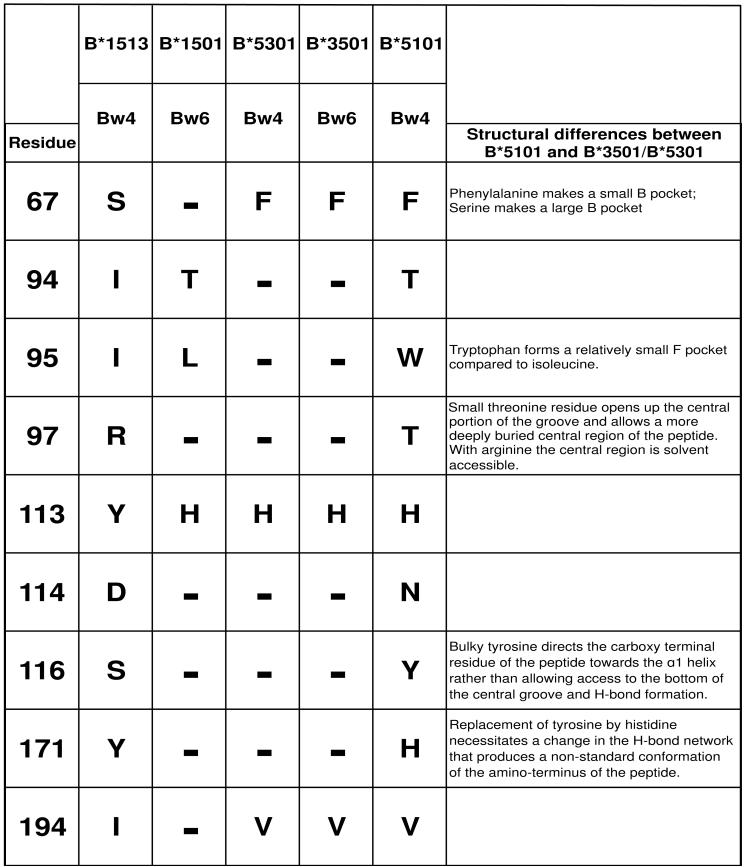
Figure 7. Changes in the B and F pockets that bind peptide anchor residues are correlated with differential KIR3DL1 engagement by B*5101-L82R and B*1513-L82R.
Shown are the nine residues that distinguish B*1513 and B*5101 and influence the binding to KIR3DL1. Also shown is their polymorphism in B*1501, B*5301 and B*3501. Identities with B*1513 are shown by a dash. B*5301 and B*3501 differ only in the Bw4/Bw6 motif. Crystallographic structures have been determined for all these allotypes, except B*1513 (27, 42-44). Although B*5101 has serological similarities with B*5301 and B*3501 it has an unusual peptide binding-site as summarised under ‗Structural differences between B*5101 and B*5301/B*3501’. At the key positions that cause these differences (67, 95, 97, 116 and 171) B*1513 is identical to B*3501. Thus the peptide binding site of B*1513 is likely to be more like those of B*5301 and B*3501, and distinct from that of B*5101.