Abstract
Carbon monoxide (CO), a by-product released during the degradation of heme by heme oxygenases (HOS EC 1.14.99.3) in animals, plays a major role as neurotransmitter, regulator of sinusoidal tone, inhibitor of platelet aggregation and suppressor of acute hypertensive response, and most of above effects are similar to or mediated by nitric oxide (NO), another signal molecule in both the animal and plant kingdoms. Previous result demonstrated that NO could act as a promoter of plant cell elongation, acting similarly to IAA, inducing morphogenetic responses leading to expansion in plant tissues. Recent observations revealed that CO is an inducer of cell expansion in wheat root segments, acting similarly to IAA and NO. Evidence also indicated that IAA could result in either the potent induction of HO-1 transcript or endogenous CO releasing in wheat root segments. Additionally, our results suggested that above CO signaling might be related to NO/cGMP, Ca2+ and even ROS-dependent pathways. In this addendum, combined with other previous results, we further proposed a possible hypothesis for CO signaling role in regulation of plant root development induced by auxin.
Key words: auxin, carbon monoxide, nitric oxide, plants, reactive oxygen species, signal molecule
Carbon monoxide (CO) is a low molecular weight diatomic gas that occurs ubiquitously in nature as an air pollutant. In plants, although several laboratories1,2 have reported the direct emission of CO by living plants since 1959, little information is known about CO's physiological roles in the whole plant, except that exogenous CO was able to affect the plant seed germination, induce the adventitious rooting process.3,4 While in animals, CO which was mainly produced by heme oxygenase (HO EC 1.14.99.3), has recently seen an explosion of research interest due to its newly discovered physiological and signaling effects. In animals, there are three forms of HOs. HO-1 is inducible, while constitutively expressed HO-2 and HO-3 display very low activity. Research in CO now embraces the entire field of medicine where reactive oxygen species (ROS), reactive nitrogen species (RNS), inflammation, growth control and apoptosis represent important pathophysiological mechanisms.5–7 Meanwhile, downstream signaling events regulated by CO in animals have been described before.7,8 Among the second messengers reported to be involved in CO signaling, there are at least cyclic GMP (cGMP), nitric oxide (NO) and ROS, but in plants this study is also in its infancy.
It is well known that the plant hormone auxin is involved in the regulation of most aspects of plant growth and development processes, including cell division, elongation and differentiation. It has been demonstrated that endogenous NO is involved in IAA-mediated root organogenesis.9-11 The use of CO gas, or heme molecules hematin and hemin, also termed as HO inducer to yield CO in animals, has shown that exogenous CO is involved in adventitious rooting and lateral root formation.12,13 In our study, we report that administration of hematin and hemin, exactly induced the significant increase in wheat root elongation as well as the actions of IAA and NO donor sodium nitroprusside (SNP) in a dose-dependent manner.14 These responses were mimicked by the application of aqueous solution of CO with different saturation. Additionally, above heme molecule-induced effect is specific for CO produced by HO since the potent inhibitor of HO-1, zinc protoporphyrin-IX (ZnPPIX) or CO/NO scavenger hemoglobin (Hb) blocked the action of hematin and hemin, respectively. Our further results also confirmed that hematin could result in either the potent induction of HO-1 transcript or endogenous CO releasing in the wheat root tip segments. In contrast, when ZnPPIX was added together, the increase of HO-1 transcript or CO content was reversed.
Exogenously applied CO may not replicate the function of endogenous CO and may have side effects in plants. More recently, we also discovered15 that under 100 and 200 µM cadmium treatments, CO releasing was increased and approximately consistent with the changes of HO activity and HO-1 transcript, an important CO synthetic enzyme both in animals and plants.16 In Vicia faba leaves, CO production and HO activity were firstly reported to increase in response to ABA treatment, which could result in stomatal closure. Interestingly, ABA-induced stomatal closure in Vicia faba guard cells was differentially blocked when ZnPPIX or Hb was added.17 In our further experiments, our data presented that treatment with IAA could result in either the potent induction of HO-1 transcript or CO releasing in wheat root segments. ZnPPIX with lower concentration could prevent the elongation induced by IAA, while in the SNP-treatment the prevention of root growth occurred solely at higher concentrations. By using specific histochemical assay combined with the inhibitor investigation, we also suggested that endogenous CO generated by HO might mediate the induction of growth elongation of wheat root segments elicited by IAA, which might be also related to NO/cGMP, Ca2+ and even ROS-dependent pathways.14
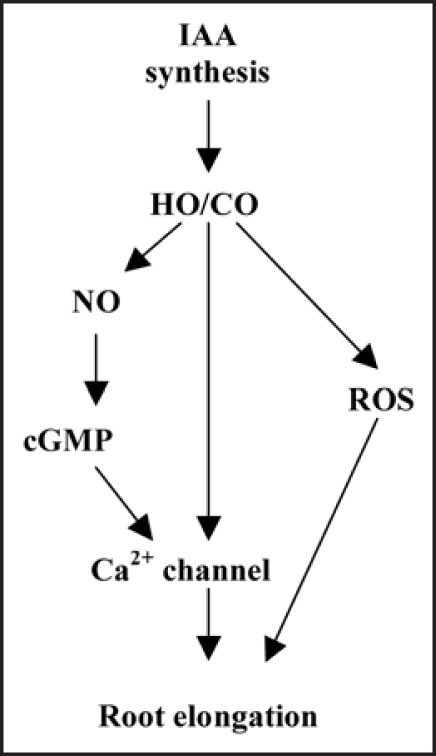
Furthermore, we advanced a simple possible signaling pathway accounting for findings showed in this paper (Fig. 1). In this model, endogenous CO, generated from HO acting as a potent signal molecule defines an underlying link between the upstream hormone IAA and the downstream signal NO, thus leading to wheat root elongation. Additionally, cGMP and ROS might also be related to above signal transduction. Considering that CO has been postulated as a possible signal molecule during development and adaptive plant response against some abiotic stresses,15,17 our results further suggest that CO might be as a versatile molecule with different functions in plants, which was proven in animal systems recently. Additionally, functional studies will be directed to evaluate the participation of CO on auxin-mediated processes using mutants unpaired in CO production, thus leading to full understanding the role of auxin-induced and CO-mediated signaling pathways in plants.
Figure 1.
Schematic illustration of a proposed model for HO/CO signaling pathway of root elongation in wheat. IAA triggers a transient CO accumulation, which activates an NO or ROS-dependent pathway, thus leading to root elongation.
Acknowledgements
This work was supported by the Program for New Century Excellent Talents in University, the National Natural Science Foundation of China (grant no. 30671248), the Natural Science Foundation of Jiangsu Province of China (grant no. BK2007157), the 111 Project (grant no. B0703), and Student Research Training (SRT) Project (grant no. 0506B07) of Nanjing Agricultural University, China.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/5374
References
- 1.Wilks SS. Carbon monoxide in green plants. Science. 1959;129:964–966. doi: 10.1126/science.129.3354.964. [DOI] [PubMed] [Google Scholar]
- 2.Lüttge U, Fischer K. Light-dependent net CO-evolution by C3 and C4 plants. Planta. 1980;149:59–63. doi: 10.1007/BF00386228. [DOI] [PubMed] [Google Scholar]
- 3.Zimmerman PW, Crocker W, Hitchcock AE. Initiation and stimulation of roots from exposure of plants to carbon monoxide gas. Contrib Boyce Thompson Inst. 1933;5:1–17. [Google Scholar]
- 4.Liu KL, Xu S, Xuan W, Ling TF, Cao ZY, Huang BK, Sun YG, Fang L, Liu ZY, Zhao N, Shen WB. Carbon monoxide counteracts the inhibition of seed germination and alleviates oxidative damage caused by salt stress in Oryza sativa. Plant Sci. 2007;172:544–555. [Google Scholar]
- 5.Dulak JJ, Józkowicz A. Carbon monoxide—a ‘new’ gaseous modulator of gene expression. Acta Biochim Pol. 2003;50:31–47. [PubMed] [Google Scholar]
- 6.Piantadosi CA. Biological chemistry of carbon monoxide. Antiox Redox Signal. 2002;4:259–270. doi: 10.1089/152308602753666316. [DOI] [PubMed] [Google Scholar]
- 7.Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: a putative neural messenger. Science. 1993;259:381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- 8.Hartsfield CL. Cross talk between carbon monoxide and nitric oxide. Antiox Redox Signal. 2002;4:301–307. doi: 10.1089/152308602753666352. [DOI] [PubMed] [Google Scholar]
- 9.Pagnussat GC, Simontacchi M, Puntarulo S, Lamattina L. Nitric oxide is required for root organogenesis. Plant Physiol. 2002;129:954–956. doi: 10.1104/pp.004036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pagnussat GC, Lanteri ML, Lamattina L. Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiol. 2003;132:1241–1248. doi: 10.1104/pp.103.022228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Correa-Aragunde N, Graziano M, Lamattina L. Nitric oxide plays a central role in determining lateral root development in tomato. Planta. 2004;218:900–905. doi: 10.1007/s00425-003-1172-7. [DOI] [PubMed] [Google Scholar]
- 12.Xu J, Xuan W, Huang BK, Zhou YH, Ling TF, Xu S, Shen WB. Carbon monoxide-induced adventitious rooting of hypocotyls cutting from mung bean seedling. Chin Sci Bull. 2006;51:668–674. [Google Scholar]
- 13.Cao ZY, Xuan W, Liu ZY, Li XN, Zhao N, Xu P, Wang Z, Guan RZ, Shen WB. Carbon monoxide promotes lateral root formation in rapeseed. J Integr Plant Biol. 2007;49:1007–1016. [Google Scholar]
- 14.Xuan W, Huang LQ, Li M, Huang BK, Xu S, Liu H, Gao Y, Shen WB. Induction of growth elongation in wheat root segments by heme molecules: a regulatory role of carbon monoxide in plants? Plant Growth Regul. 2007;52:41–51. [Google Scholar]
- 15.Han Y, Zhang J, Chen XY, Gao ZZ, Xuan W, Xu S, Ding X, She WB. Carbon monoxide alleviates cadmium-induced oxidative damage by modulating glutathione metabolism in the roots of Medicago sativa. New Phytol. 2008;177:155–166. doi: 10.1111/j.1469-8137.2007.02251.x. [DOI] [PubMed] [Google Scholar]
- 16.Muramoto T, Tsurui N, Terry MJ, Yokota A, Kohchi T. Expression and biochemical properties of a ferredoxin-dependent heme oxygenase required for phytochrome chromophore synthesis. Plant Physiol. 2002;130:1958–1966. doi: 10.1104/pp.008128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao ZY, Huang BK, Wang QY, Xuan W, Ling TF, Zhang B, Chen X, Nie L, Shen WB. Involvement of carbon monoxide produced by heme oxygenase in ABA-induced stomatal closure in Vicia faba and its proposed signal transduction pathway. Chin Sci Bull. 2007;52:2365–2373. [Google Scholar]