Abstract
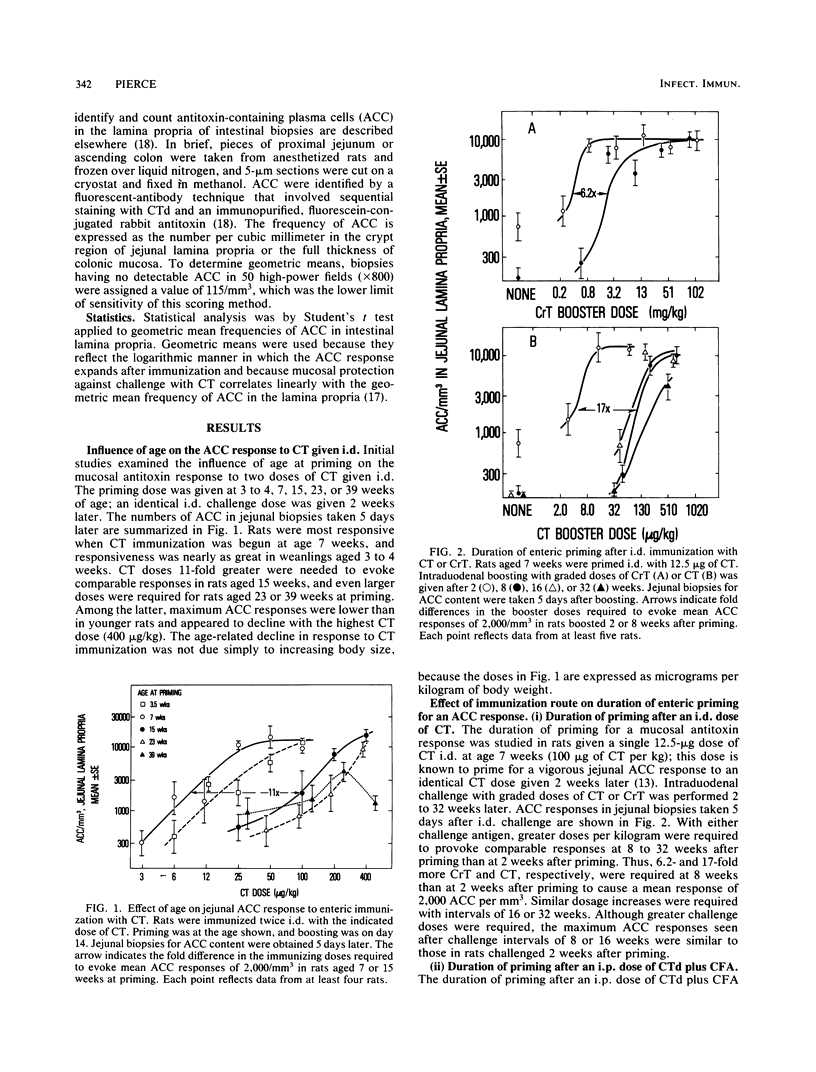
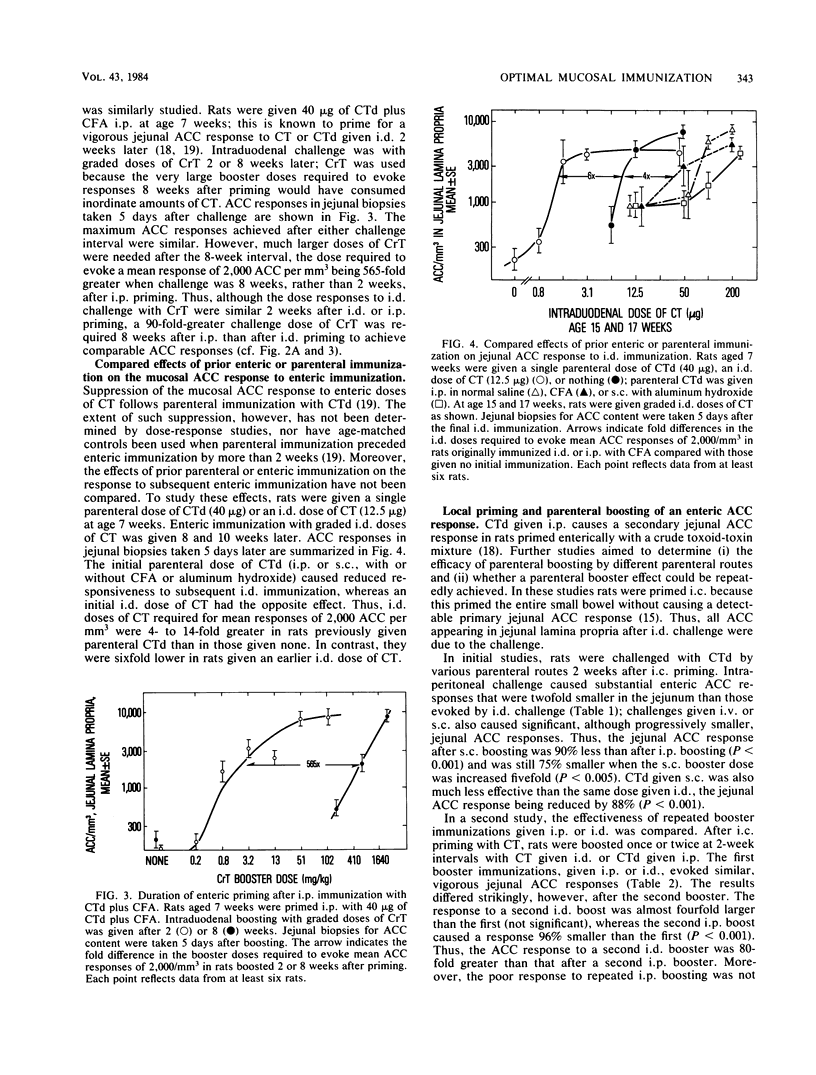
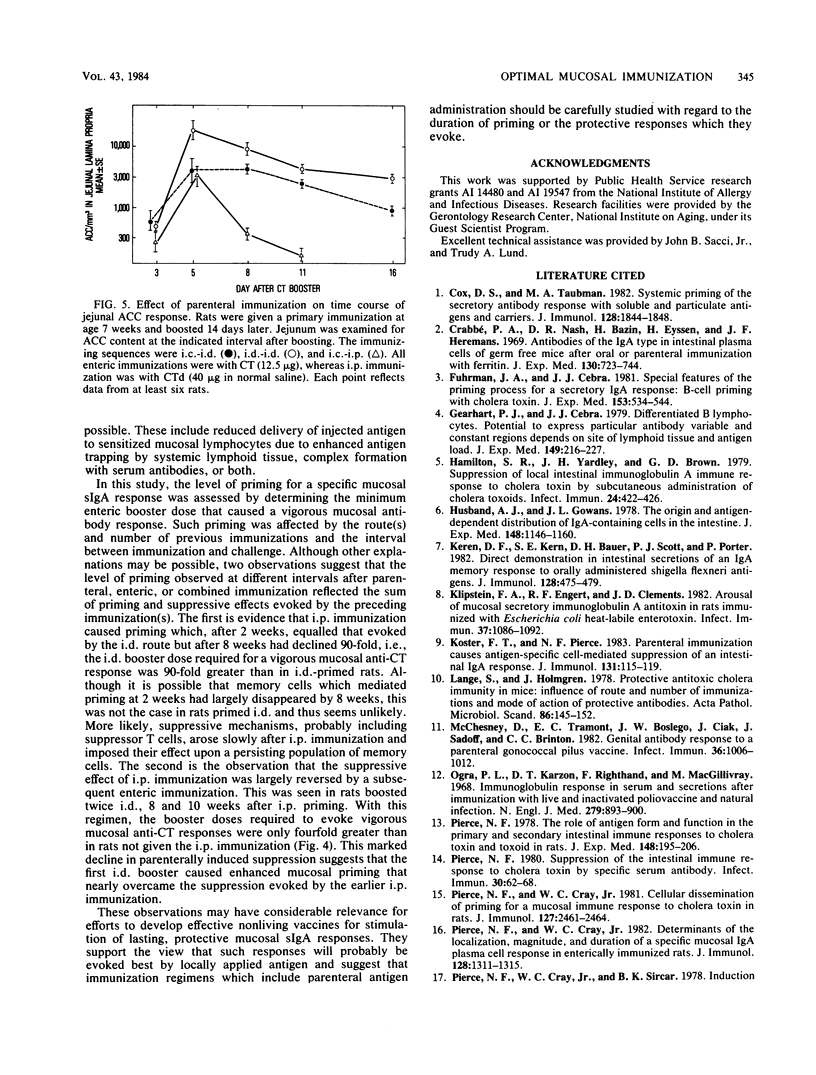
The antitoxin response in intestinal mucosa was studied in rats immunized either intestinally or by combined parenteral and intestinal dosing with cholera toxin or cholera toxoid. Attention was given to the duration of enteric priming and the magnitude and time course of mucosal anti-cholera toxin responses in rats of defined age. Cholera toxin given only intraduodenally was a more efficient priming immunogen in young rats than in older rats and caused priming that lasted at least 32 weeks; repeated enteric doses increased local priming and repeatedly evoked vigorous mucosal anti-cholera toxin responses which occurred rapidly and declined slowly. Results differed when a portion of the immunizing regimen was parenteral. Cholera toxoid given intraperitoneally (i.p.) caused mucosal priming that peaked promptly and then rapidly declined; parenteral boosting after enteric priming was much more effective given i.p. than subcutaneously; moreover, the booster response was brief, virtually disappearing within 11 days, and could not be reproduced by a second i.p. immunization. These results accord with evidence that parenteral immunization both stimulates and suppresses mucosal secretory immunoglobulin A responses, whereas local immunization is not known to be suppressive. Evidence for parenterally induced suppression was the rapid decline in mucosal priming after i.p. immunization, the shortened mucosal antibody response after i.p. immunization, and possibly the inability to parenterally evoke a booster response twice. In these studies, the level of priming observed at different intervals after parenteral, enteric, or combined immunization appeared to reflect the sum of priming and suppressive effects evoked by the preceding immunization(s).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cox D. S., Taubman M. A. Systemic priming of the secretory antibody response with soluble and particulate antigens and carriers. J Immunol. 1982 Apr;128(4):1844–1848. [PubMed] [Google Scholar]
- Crabbé P. A., Nash D. R., Bazin H., Eyssen D. V., Heremans J. F. Antibodies of the IgA type in intestinal plasma cells of germfree mice after oral or parenteral immunization with ferritin. J Exp Med. 1969 Oct 1;130(4):723–744. doi: 10.1084/jem.130.4.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman J. A., Cebra J. J. Special features of the priming process for a secretory IgA response. B cell priming with cholera toxin. J Exp Med. 1981 Mar 1;153(3):534–544. doi: 10.1084/jem.153.3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhart P. J., Cebra J. J. Differentiated B lymphocytes. Potential to express particular antibody variable and constant regions depends on site of lymphoid tissue and antigen load. J Exp Med. 1979 Jan 1;149(1):216–227. doi: 10.1084/jem.149.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton S. R., Yardley J. H., Brown G. D. Suppression of local intestinal immunoglobulin A immune response to cholera toxin by subcutaneous administration of cholera toxoids. Infect Immun. 1979 May;24(2):422–426. doi: 10.1128/iai.24.2.422-426.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husband A. J., Gowans J. L. The origin and antigen-dependent distribution of IgA-containing cells in the intestine. J Exp Med. 1978 Nov 1;148(5):1146–1160. doi: 10.1084/jem.148.5.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren D. F., Kern S. E., Bauer D. H., Scott P. J., Porter P. Direct demonstration in intestinal secretions of an IgA memory response to orally administered Shigella flexneri antigens. J Immunol. 1982 Jan;128(1):475–479. [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F., Clements J. D. Arousal of mucosal secretory immunoglobulin A antitoxin in rats immunized with Escherichia coli heat-labile enterotoxin. Infect Immun. 1982 Sep;37(3):1086–1092. doi: 10.1128/iai.37.3.1086-1092.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster F. T., Pierce N. F. Parenteral immunization causes antigen-specific cell-mediated suppression of an intestinal IgA response. J Immunol. 1983 Jul;131(1):115–119. [PubMed] [Google Scholar]
- Lange S., Holmgren J. Protective antitoxic cholera immunity in mice: influence of route and number of immunizations and mode of action of protective antibodies. Acta Pathol Microbiol Scand C. 1978 Aug;86C(4):145–152. doi: 10.1111/j.1699-0463.1978.tb02572.x. [DOI] [PubMed] [Google Scholar]
- McChesney D., Tramont E. C., Boslego J. W., Ciak J., Sadoff J., Brinton C. C. Genital antibody response to a parenteral gonococcal pilus vaccine. Infect Immun. 1982 Jun;36(3):1006–1012. doi: 10.1128/iai.36.3.1006-1012.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Cray W. C., Jr Cellular dissemination of priming for a mucosal immune response to cholera toxin in rats. J Immunol. 1981 Dec;127(6):2461–2464. [PubMed] [Google Scholar]
- Pierce N. F., Cray W. C., Jr Determinants of the localization, magnitude, and duration of a specific mucosal IgA plasma cell response in enterically immunized rats. J Immunol. 1982 Mar;128(3):1311–1315. [PubMed] [Google Scholar]
- Pierce N. F., Gowans J. L. Cellular kinetics of the intestinal immune response to cholera toxoid in rats. J Exp Med. 1975 Dec 1;142(6):1550–1563. doi: 10.1084/jem.142.6.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Koster F. T. Priming and suppression of the intestinal immune response to cholera toxoid/toxin by parenteral toxoid in rats. J Immunol. 1980 Jan;124(1):307–311. [PubMed] [Google Scholar]
- Pierce N. F. Suppression of the intestinal immune response to cholera toxin by specific serum antibody. Infect Immun. 1980 Oct;30(1):62–68. doi: 10.1128/iai.30.1.62-68.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F. The role of antigen form and function in the primary and secondary intestinal immune responses to cholera toxin and toxoid in rats. J Exp Med. 1978 Jul 1;148(1):195–206. doi: 10.1084/jem.148.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm A. M., Hanson L. A., Holmgren J., Lindblad B. S., Nilsson B., Quereshi F. Different secretory immunoglobulin A antibody responses to cholera vaccination in Swedish and Pakistani women. Infect Immun. 1980 Nov;30(2):427–430. doi: 10.1128/iai.30.2.427-430.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm A. M., Lange S., Holmgren J. Intestinal immune response to cholera toxin: dependence on route and dosage of antigen for priming and boosting. Infect Immun. 1980 Nov;30(2):337–341. doi: 10.1128/iai.30.2.337-341.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


