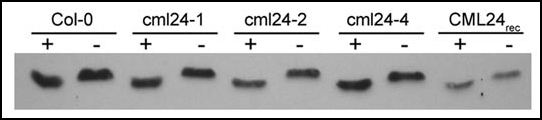
Figure 3.
Mutant CML24 proteins show a Ca2+-dependent mobility shift. Wild type (Col-0) and mutant CML24 proteins (cml24-1, cml24-2 and cml24-4) were analyzed along side purified CML24 produced heterologously in E. coli (CML24rec). Protein samples contained either 5 mM CaCl2 (+) or 5 mM EGTA (−) and were separated by 13% SDS-PAGE. The proteins were transferred to nitrocellulose membrane and probed with anti-CML24 antibody. The faster migration of wild-type, mutant, and recombinant CML24 in the presence of Ca2+ than in the presence of EGTA indicates that mutations in cml24-1, cml24-2 and cml24-4 do not completely impair Ca2+ binding and consequent conformational changes.