Figure 5.
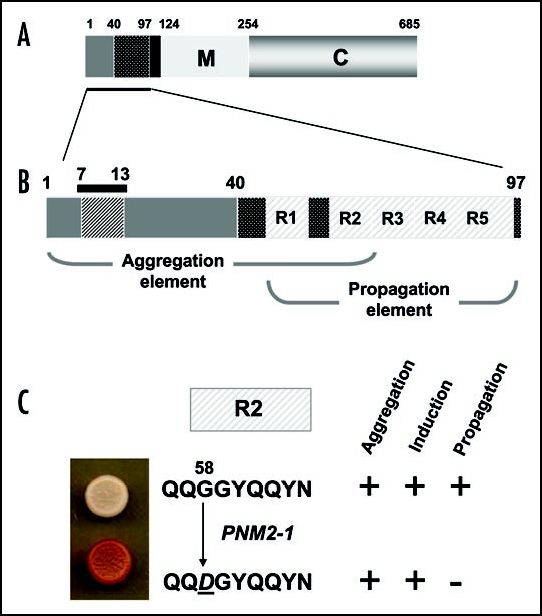
The prion-forming domain of the Sup35p protein. (A) The 685 residue Sup35p protein has three regions defined by the locations of the first three in-frame AUG (Met) codons: the amino terminal N domain (residues 1–123) that is absolutely required for the prion behaviour of the protein; the highly charged middle region (M; residues 124–253) and the carboxyl-terminal domain (C; residues 254–685) which carries the essential release factor activity.20,48 (B) Within the N domain lies a region, the so-called prion-forming domain-which contains a region important for aggregation and a separate but overlapping region important for propagation of the prion state.48 The aggregation element contains a short peptide sequence based around residues 7 to 13, that is highly amyloidogenic. The propagation element contains five imperfect copies of an oligopeptide repeat sequence (R1–R5). (C) The ‘PSI-No-More’ mutant PNM2-1 carries a single amino acid substitution (Gly to Asp) within repeat R2 of the propagation element. This single substitution leads to a form of Sup35p that can still aggregate but can no longer be efficiently propagated in most laboratory strains.20,48,50
