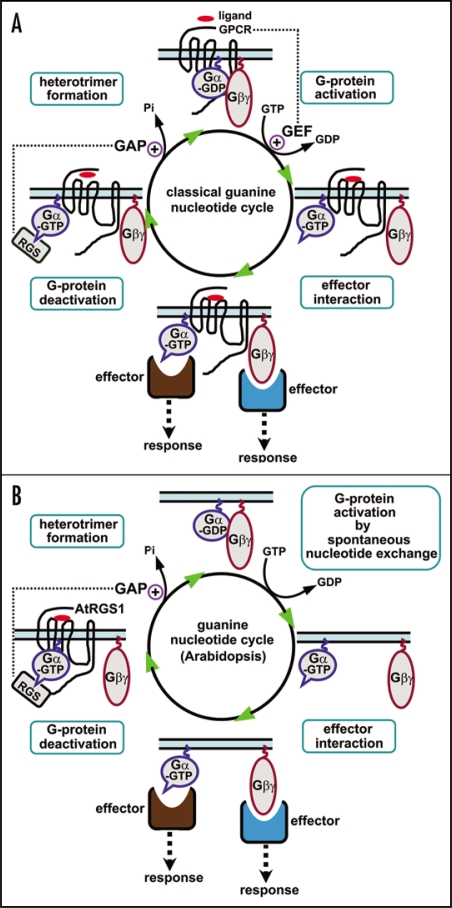
Figure 1.
Modes of action of the heterotrimeric G-proteins. (a) A classical guanine nucleotide cycle of heterotrimeric Gα in animals. Heterotrimeric G-proteins, composed of Gα, Gβ and Gγ subunits, couple a specific class of cell-surface receptors, seven transmembrane (7TM) G-protein-coupled receptors (GPCRs). Upon ligand occupancy, GPCRs function as guanine-nucleotide exchange factors (GEFs) to promote the exchange of GDP for GTP on Gα, which subsequently causes the dissociation of Gβγ dimer from Gα. The activated Gα (GTP-bound) and liberated Gβγ dimer can then interact with downstream effectors, resulting in corresponding cellular responses. The GTP-bound Gα is returned to its inactive form (GDP-bound) by its intrinsic GTPase activity. The Regulator of G-protein Signaling (RGS) proteins possess GTPase accelerating protein (GAP) activity on Gα. (b) Guanine nucleotide cycle of heterotrimeric Gα in Arabidopsis. Arabidopsis genome encodes one canonical Gα (GPA1), one Gβ (AGB1) and two Gγ (AGG1 and AGG2) subunits. GPA1 is an unusual heterotrimeric Gα because of a rapid nucleotide exchange and the slowest GTPase activity. Therefore, the GTP hydrolysis, rather than GDP release, is likely the rate-limiting step in the guanine nucleotide cycle of GPA1. Classical GPCRs (possessing GEF activity on Gα) may not be required for the activation of G-protein signaling because GPA1 is in the activated state (GTP-bound) by default. Instead, AtRGS1, the sole RGS protein in Arabidopsis, functions as a critical regulator of G-protein signaling by accelerating the slow, intrinsic GTPase activity of GPA1. It is likely that AtRGS1 acts as a ligand-regulated 7TM GAP for GPA1. The exact ligand for AtRGS1 that modulates GAP activity has not been identified, though D-glucose has been shown to stimulate the interaction between AtRGS1 and GPA1. Upon activation, Gα and Gβγ subunits are shown to dissociate in this model, but Arabidopsis G-proteins may signal through nondissociable heterotrimers.21