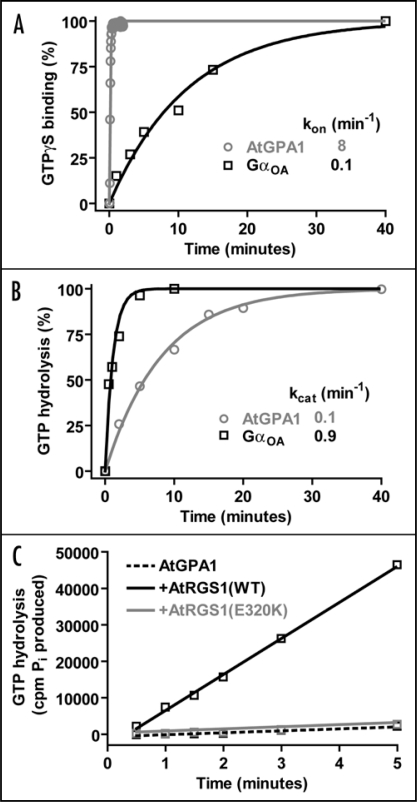
Figure 1.
Enzymological properties of the Arabidopsis heterotrimeric G-protein alpha subunit (AtGPA1) in comparison to human GαoA. (A) GTPγS binding rates of AtGPA1 and GαoA were measured using standard methods.6,20 Data were fit using a single exponential function and rate constants are presented in min−1. Note: the observed GTPγS binding rate (kon) is equivalent to the rate of GDP release, as GDP release is the rate limiting step in this process.11 (B) The GTP hydrolysis rates of AtGPA1 and GαoA were measured using single turnover GTP hydrolysis78 (i.e., using Gα subunits preloaded with [γ-32P]GTP). Data were fit using a single exponential function and rate constants are presented in min−1. (C) Steady state GTP hydrolysis of 100 nM AtGPA1 was measured using [γ-32P]GTP, as described.6 AtGPA1 was incubated with either buffer (dotted black line), 250 nM wild-type AtRGS1(black line), or 250 nM E320K AtRGS1(grey line). The latter protein serves as a negative control for this experiment as it harbors a glutamate-320 to lysine (E320K) charge-reversal mutation that cripples RGS domain-mediated GAP activity, as previously described.6,79 A general phenomenon of RGS proteins is that steady state GTPase acceleration cannot be observed in the absence of a GEF80 as GDP release (not GTP hydrolysis) is rate limiting step in the G-protein cycle.11 However, in this case, the steady state GTPase rate of AtGPA1 is greatly accelerated in the presence of AtRGS1-mediated GAP activity, highlighting the fact that GDP release is not rate-limiting for this plant Gα subunit. Observed rate constants were calculated by linear regression: AtGPA1 532 cpm/min, + wild-type AtRGS1 9867 cpm/min, + E320K AtRGS1 579 cpm/min.