Abstract
The recent cloning of the pea genes LA and CRY has historical implications, since the combined effect of null mutations in these genes is the elongated, gibberellin-insensitive “slender” phenotype, which gave rise to the theory that gibberellins (GAs) are inhibitors of inhibitors of growth. Interestingly, the duplication event that produced the second gene (LA or CRY) appears to have occurred more than 100 mya, and yet the two genes have retained essentially similar functions. They both encode DELLA proteins, which inhibit growth while at the same time promoting the synthesis of the growth-promoting hormone, gibberellin (GA). This duality of function is discussed in the context of recent suggestions that DELLAs integrate multiple hormone signals, rather than just the GA signal. We also present new data showing that LA and CRY play a major role in regulating fruit growth.
Key words: DELLA proteins, gibberellin, slender phenotype, pea, elongation
Historical Aspects
We recently reported the cloning of the pea genes LA and CRY, showing that they encode DELLA proteins.1 These proteins are inhibitors of stem elongation, and are destabilised by bioactive gibberellins (GAs).2 Thus the GAs are promoters of growth because they are inhibitors of inhibitors of growth, an idea suggested by Percy Brian in 1957,3 on the basis of the elongated la cry-s (“slender”) pea phenotype. Brian's perspicacity is now fully vindicated by the characterisation of LA and CRY.
The genes LA and CRY were among the first to be described as “duplicate genes”.4 The concept of duplicate genes originated soon after the rediscovery of Mendel's laws of inheritance in the early 1900s; perhaps the earliest mention is by Shull,5 referring to genes controlling fruit shape in Capsella bursa-pastoris (shepherd's purse). The idea that two independent genes might encode a very similar protein was vindicated much later, during the era of modern molecular biology, by the discovery of gene families.
Interestingly, until recently the fate of duplicate genes was considered to be either loss of one gene or the acquisition of a new role for one gene.6 Clearly, neither scenario has occurred in the case of the two DELLA-encoding genes from pea.1 This would not be surprising if the duplication that led to LA and CRY had occurred recently, but phylogenetic analysis suggests it is probably ancient, preceding the divergence of the lineages leading to pea and Arabidopsis.1 Thus LA and CRY provide an interesting example of duplicate genes that appear to have persisted with similar roles for considerable time in evolutionary terms (117–108 million years ago7).
We should add, however, that while the functions of LA and CRY are essentially similar in terms of shoot elongation (and in fact, on an otherwise wild-type background, la CRY plants are indistinguishable from LA cry-s plants), the contribution of LA to the inhibition of root elongation appears to outweigh that of CRY.1 Furthermore, we are still in the process of dissecting out other, possibly differing, effects of these genes on the overall architecture of the shoot.
DELLAs as Integrators of Multiple Signals?
In addition to the control of elongation growth, another function performed by both LA and CRY is the regulation of GA biosynthesis and deactivation.1 Both these proteins upregulate the expression of GA synthesis genes and downregulate the expression of GA deactivation (2-oxidase) genes. In other words, somewhat paradoxically, DELLA protein activity results in both an accumulation of bioactive GAs and an inhibition of growth. The effect of DELLAs on GA synthesis gene expression in shoots has been known for some time,8–10 and has now been extended to the roots.1 Clearly, this property of DELLAs has the potential to link factors affecting DELLA stability with the regulation of GA biosynthesis, and indeed it is through this mechanism that GAs regulate their own levels.
Until 2003, the GAs were the only factor thought to affect DELLA stability. Then Fu and Harberd11 suggested that in Arabidopsis roots auxin also affects DELLA stability, or at least, that auxin is required for GA to destabilise DELLAs. Since that time, several papers have implicated other hormones, in particular ethylene and abscisic acid (ABA), in the regulation of DELLA stability and therefore of DELLA levels; these latter two hormones are suggested to stabilise the DELLAs.12–14 Thus, it has been suggested that DELLA proteins are key integrators of several hormonal signals, of which GA is only one.
However, this conceptual shift should take into account the finding that DELLAs are promoters of GA synthesis. The key point is that factors that affect DELLA stability will also tend to affect GA accumulation. A stabilisation of DELLAs by ABA, for example, might tend to upregulate GA synthesis, especially since DELLAs appear to directly affect that process.15 Interestingly, however, it is also suggested that ABA downregulates GA synthesis.15 Determining the outcome of such “tugs-of-war” between opposing effects will be central to our understanding of hormone interactions and their role in plant growth.
It should be noted, however, that at this stage the status of DELLAs as integrators of multiple signals is not beyond question. The suggestion that ABA stabilises DELLAs13 has been called into question by some authors;5 see also.16,17 Further, in decapitated pea stems, applied GA can cause a strong growth response, and by implication can effectively destabilise DELLAS, even though auxin levels are dramatically reduced.18 This indicates that in peas auxin might not be required for DELLA destabilisation.
DELLAs and Reproductive Development
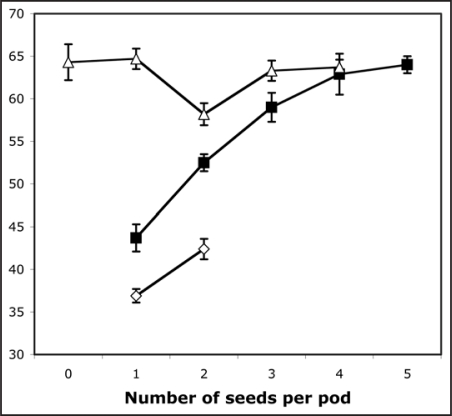
While the DELLA proteins in pea exert major effects on shoot and root elongation,1,19 their role in the growth of other organs (e.g., fruit) is less clearly defined. It has been reported that la cry-s plants show poor seed-set and parthenocarpic pod development.4 The reason for the poor seed set is not clear at present, but a similar phenomenon is known to occur in other DELLA-deficient mutants.20 Interestingly, however, we have observed that parthenocarpic pods of slender plants undergo normal elongation (Figs. 1 and 2). In peas, pod length is usually related to the number of seeds in the pod.21 This relationship also holds in GA-deficient mutants such as Mendel's le-1 dwarf and in the extreme dwarf na-1 mutant, but not in la cry-s slender plants (Fig. 2). This clearly shows that DELLA proteins are also inhibitors of pod elongation and suggests that LA and CRY are the only DELLAs that control pod elongation in pea.
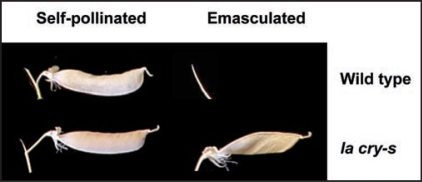
Figure 1.
Pods of the la cry-s slender mutant elongate strongly even when emasculated to prevent self-pollination. In contrast, emasculating wild-type flowers usually results in abscission of the entire flower, before pod elongation, leaving only the peduncle, as shown. If parthenocarpic pods do develop on the wild-type, they are very small.
Figure 2.
Pod length plotted against seed number for self-fertilised pods developing on slender (la cry-s, ▵), dwarf (le-1, ▪) and nana (na-1, ♦) plants from a segregating progeny. The number of pods in each sample were (for slender, left to right) 9, 3, 5, 3, 3; for dwarf, 14, 25, 15, 7, 2; and for nana, 65, 19.
It also implies that the effect of seed number on pod length is caused/mediated by bioactive GAs, although genetic studies, combined with measurements of GA levels in pods, appear to argue against the idea that seeds directly export GAs into the pods.22,23 Although the possibility of GA export from seeds into siliques has been raised yet again recently,24 we consider it more likely that seeds export a factor such as the auxin 4 Cl-IAA, which maintains bioactive GA levels in pods, as suggested previously.25 It is interesting to note that, compared with the wild type, the elongation of le-1 pods is essentially normal.26 However, when the pods are grossly GA-deficient, as in the na-1 mutant, elongation is substantially reduced (Fig. 2), although it is not yet known whether this is a direct effect, or an indirect effect of the small stature and therefore low assimilate production of na-1 plants.
Conclusion
In conclusion, the molecular cloning of LA and CRY opens the way for further studies on hormone interactions in pea, and on how these interactions might regulate diverse developmental phenomena.
Acknowledgements
We thank Steve Swain for helpful discussions and Jennifer Smith for figure preparation.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/6224
References
- 1.Weston DE, Elliott RC, Lester DR, Rameau C, Reid JB, Murfet IC, Ross JJ. The pea DELLA proteins LA and CRY are important regulators of gibberellin synthesis and root growth. Plant Physiology. 2008;147:199–205. doi: 10.1104/pp.108.115808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harberd NP, King KE, Carol P, Cowling RJ, Peng J, Richards DE. Gibberellin: inhibitor of an inhibitor of…? BioEssays. 1998;20:1001–1008. doi: 10.1002/(SICI)1521-1878(199812)20:12<1001::AID-BIES6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 3.Brian PW. The effects of some microbial metabolic products on plant growth. Symp Soc Exp Biol. 1957;11:166–181. [PubMed] [Google Scholar]
- 4.de Haan H. Length-factors in Pisum. Genetica. 1927;9:481–498. [Google Scholar]
- 5.Shull GH. Duplicate genes for capsule-form in Bursa bursa-pastoris. Mol Gen Genet. 1914;12:97–149. [Google Scholar]
- 6.Moore RC, Purugganan MD. The evolutionary dynamics of plant duplicate genes. Curr Opin Plant Biol. 2005;8:122–128. doi: 10.1016/j.pbi.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Wikström N, Savolainen V, Chase MW. Angiosperm divergence times: congruence and incongruence between fossils and sequence divergence estimates. In: Donoghue P C J, Smith M P, editors. Telling the Evolutionary Time: Molecular Clocks and the Fossil Record. Boca Raton, USA: CRC Press; 2003. pp. 142–165. [Google Scholar]
- 8.Richards DE, King KE, Ait-Ali T, Harberd NP. How gibberellin regulates plant growth and development: A molecular genetic analysis of gibberellin signaling. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:67–88. doi: 10.1146/annurev.arplant.52.1.67. [DOI] [PubMed] [Google Scholar]
- 9.Silverstone AL, Jung H, Dill A, Kawaide H, Kamiya Y, Sun T. Repressing a repressor: gibberellin-induced rapid reduction of the RGA protein in Arabidopsis. Plant Cell. 2001;13:1555–1565. doi: 10.1105/TPC.010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun TP, Gubler F. Molecular mechanism of gibberellin signaling in plants. Annu Rev Plant Biol. 2004;55:197–223. doi: 10.1146/annurev.arplant.55.031903.141753. [DOI] [PubMed] [Google Scholar]
- 11.Fu X, Harberd NP. Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature. 2003;421:740–743. doi: 10.1038/nature01387. [DOI] [PubMed] [Google Scholar]
- 12.Achard P, Vriezen WH, Van Der Straeten D, Harberd NP. Ethylene regulates arabidopsis development via the modulation of DELLA protein growth repressor function. Plant Cell. 2003;15:2816–2825. doi: 10.1105/tpc.015685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Van Der Straeten D, Peng J, Harberd NP. Integration of plant responses to environmentally activated phytohormonal signals. Science. 2006;311:91–94. doi: 10.1126/science.1118642. [DOI] [PubMed] [Google Scholar]
- 14.Alvey L, Harberd NP. DELLA proteins: integrators of multiple plant regulatory inputs? Physiol Plant. 2005;123:153–160. [Google Scholar]
- 15.Zentella R, Zhang Z, Park M, Thomas SG, Endo A, Murase K, Fleet CM, Jikumaru Y, Nambara E, Kamiya Y, Sun T. Global analysis of DELLA direct targets in early gibberellin signaling in Arabidopsis. Plant Cell. 2007;19:3037–3057. doi: 10.1105/tpc.107.054999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bassel GW, Mullen RT, Bewley JD. procera is a putative DELLA mutant in tomato (Solanum lycopersicum): effects on the seed and vegetative plant. J Exp Bot. 2008;59:585–593. doi: 10.1093/jxb/erm354. [DOI] [PubMed] [Google Scholar]
- 17.Gubler F, Chandler PM, White RG, Llewellyn DJ, Jacobsen JV. Gibberellin signaling in barley aleurone cells. Control of SLN1 and GAMYB expression. Plant Physiol. 2002;129:191–200. doi: 10.1104/pp.010918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ross JJ, O'Neill DP, Wolbang CM, Symons GM, Reid JB. Auxin-gibberellin interactions and their role in plant growth. J Plant Growth Regul. 2002;20:346–353. doi: 10.1007/s003440010034. [DOI] [PubMed] [Google Scholar]
- 19.Potts WC, Reid JB, Murfet IC. Internode length in Pisum. Gibberellins and the slender phenotype. Physiol Plant. 1985;63:357–364. [Google Scholar]
- 20.Ikeda A, Ueguchi-Tanaka M, Sonoda Y, Kitano H, Koshioka M, Futsuhara Y, Matsuoka M, Yamaguchi J. slender rice, a constitutive gibberellin response mutant, is caused by a null mutation of the SLR1 gene, an ortholog of the height-regulating gene GAI/RGA/RHT/D8. Plant Cell. 2001;13:999–1010. doi: 10.1105/tpc.13.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozga JA, Brenner ML, Reinecke DM. Seed effects on gibberellin metabolism in pea pericarp. Plant Physiol. 1992;100:88–94. doi: 10.1104/pp.100.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Potts WC. Gibberellins in light-grown shoots of Pisum sativum L. and the influence of reproductive development. Plant Cell Physiol. 1986;27:997–1003. [Google Scholar]
- 23.MacKenzie-Hose AK, Ross JJ, Davies NW, Swain SM. Expression of gibberellin mutations in fruits of Pisum sativum L. Planta. 1998;204:397–403. [Google Scholar]
- 24.Hu J, Mitchum MG, Barnaby N, Ayele BT, Ogawa M, Nam E, Lai WC, Hanada A, Alonso JM, Ecker JR, Swain SM, Yamaguchi S, Kamiya Y, Sun TP. Potential sites of bioactive gibberellin production during reproductive growth in Arabidopsis. Plant Cell. 2008;20:320–336. doi: 10.1105/tpc.107.057752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Huizen R, Ozga JA, Reinecke DM. Seed and hormonal regulation of gibberellin 20-oxidase expression in pea pericarp. Plant Physiol. 1997;115:123–128. doi: 10.1104/pp.115.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santes CM, Hedden P, Sponsel VM, Reid JB, Garcia Martinez JL. Expression of the le mutation in young ovaries of Pisum sativum and its effect on fruit development. Plant Physiol. 1993;101:759–764. doi: 10.1104/pp.101.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]