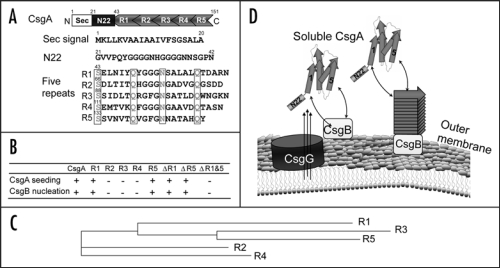
Figure 1.
R1 and R5 play important roles in guiding CsgB-responsiveness and CsgA seeding. (A) A schematic of the CsgA primary structure, including a Sec signal sequence (which is cleaved after translocation across the cytoplasmic membrane), N-terminal 22 residues involved in secretion through the outer membrane and five repeating units comprising a protease-resistant amyloid core. The regularly spaced Ser, Gln and Asn residues in each repeating unit are enclosed in boxes. (B) A summary of the interactions between CsgA and CsgB (heteronucleation), or CsgA derivatives (seeding). A “+” means that the interaction reduces the CsgA lag phase. A “−” means that the interaction has little or no affect on the CsgA lag phase. CsgA seeding was measured as previously described11 and CsgB heteronucleation was measured by the overlay assay.10 (C) Phylogram of consensus sequences (SerX5GlnX4AsnX5Gln) of five repeating units. The sequence similarity and identity between consensus sequence of R3 and R5 are higher than those between R1 and R5. (D) The nucleation model of CsgA in vivo polymerization. Curli assembly is governed by surface-localized CsgB (heteronucleation) and by the growing fiber tip (homonucleation). CsgG is the major component of the curli secretion apparatus that directs CsgA and CsgB across the outer membrane. After secretion from the periplasm to extracellular space, R1 and R5 of CsgA can interact with CsgB or fiber tips, initiating curli formation.