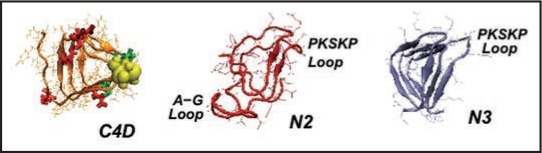
Figure 5.
Left handed β helical structures for model prion tetramers. C4: 4 turn β helix for the C-terminal region (residues 166–226). Note, the cysteines highlighted with yellow spheres, glutamates highlighted in red, and glycans linked asparagines highlighted in green. N3: 3-turn β helix for the N-terminal region drawn from references 7 and 11. N2: New 2-turn N-terminal beta helix. Images produced with VMD.67 Note: PSKPK denotes the small hinge region hypothesized to link the domain swap between tetramers; G-A represents the hydrophobic loop removed from N3 to stabilize N2.