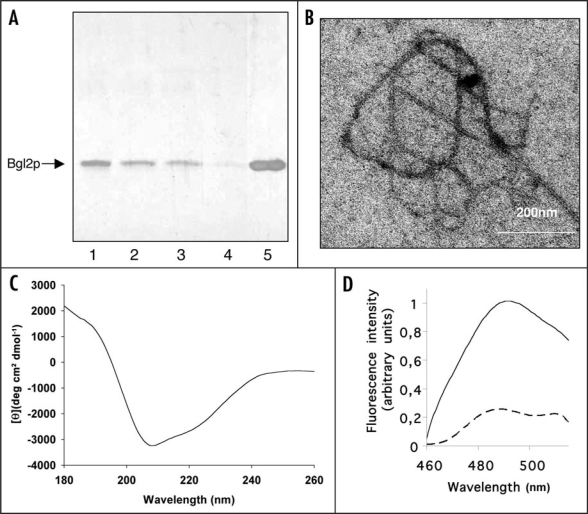
Figure 2.
Amyloid-like properties of Bgl2p. (A) SDS-PAGE analysis Bgl2p. Bgl2p was extracted from cell wall preparations as described in Materials and Methods. Proteins were visualized by silver staining. After extraction Bgl2p samples were incubated at room temperature for 0 (lane 1), 1 hour (lane 2), 24 hours (lane 3) and 96 hours (lane 4) and applied to SDS-PAGE without boiling after addition of Laemmli buffer. In lane 5 the Bgl2p sample identical to that applied to lane 4 was boiled after buffer addition (5 min, 100°C). (B) Electron microscopy analysis of Bgl2p samples. Bgl2p sample were incubated at room temperature for 96 hours and inspected by electron microscopy. Bgl2p is present as high molecular weight structures forming fibrils (for control we executed parallel experiments using cell wall preparations of the Δbgl2 strain, no fibril-like structures were observed in such control experiments). Negative-staining EM was used. (C) Circular dichroism spectrum of Bgl2p at 20°C. Bgl2p was extracted from cell wall as described in Materials and Methods. The spectrum revealed the high β-sheet structure content in the sample. (D) Fluorescence emission spectra of Bgl2p-bound thioflavin T. Bgl2p was extracted from cell wall preparations (solid line) and the identical sample was boiled for 5 minutes before applying (dashed line). Autofluorescence of protein in the absence of ThT was negligible. Excitation wavelength is 435 nm.