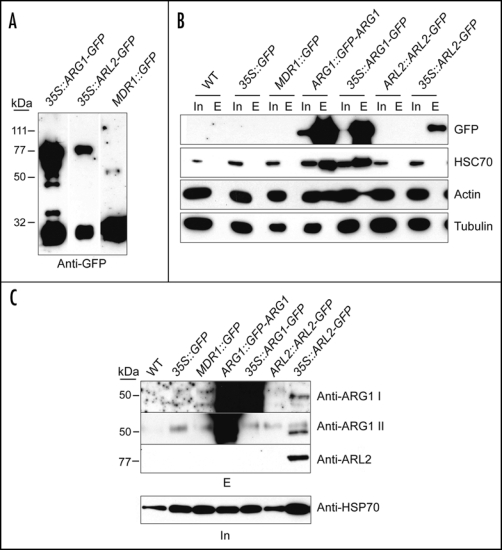
Figure 1.
HSC70, actin and ARL2 co-purify with ARG1 from plant extracts. Total protein extracts from four-week-old liquid grown plants of the indicated genotypes (top) were affinity-purified using anti-GFP antibodies (gift of Richard Vierstra, UW-Madison) cross-linked to Protein A-Sepharose beads and detected with the indicated antibodies. In (A) the affinity-purified eluate was probed with anti-GFP antibody to indicate the presence of both full-length GFP fusions and breakdown products. In (B) western blots of total protein extracts (Input, In) and affinity-purified eluates (E) were probed with anti-HSC70 (diluted 1:10,000; Stressgen, San Diego, CA), monoclonal anti-actin (diluted 1:1000; clone C4 from Chemicon Int., Temecula, CA), or anti-a-tubulin antibodies (Sigma, St Louis, MO). In (C) western blots of affinity-purified eluates (top) were probed with two anti-ARG1 antibodies raised in rabbits against a bacterially expressed His6-ARG1 protein fusion (anti-ARG1 I) and against a peptide corresponding to the 15 C-terminal residues of ARG1 (anti-ARG1 II), both used at 1:1000 dilutions, as well as with a polyclonal anti-ARL2 antibody raised in rabbits against a synthetic ARL2 peptide, used at 1:3000 dilution. Note that anti-ARG1 II did not recognize the ARG1-GFP protein probably because the corresponding C-terminal epitope was masked in this C-terminal fusion. A western blot of total protein extracts used for affinity purification (In, bottom) was probed with anti-HSP70 antibody (gift of Elizabeth Craig, UW-Madison; 1:20,000 dilution) to show relative amounts of protein prior to extraction. Experimental procedures. Plants expressing ARG1::GFP-ARG1 and 35S::ARL2-GFP are described elsewhere (reviewed in refs. 4 and 6, respectively). Plants expressing 35S::ARG1-GFP were generated by transformation with a T-DNA derived from the binary vector pK7FWG225 containing the ARG1 cDNA, whereas plants expressing AtMDR1::GFP were a gift from Edgar Spalding (UW-Madison). For immunoprecipitation, anti-GFP serum was cross-linked to Protein A Sepharose (Amersham, Piscataway, NJ) using the cross-linking reagent disuccinimidyl suberate according to the manufacturer's recommendations (Pierce, Rockford, Ill.). Extracts from liquid-grown plants were generated by grinding in 1:1 (vol:wt) ice cold grinding buffer [20 mM Tris, 1 mM EDTA, 100 mM NaCl, 0.1% Triton X-100 pH7.5, containing 1:100 dilution of protease inhibitor cocktail (Sigma, or Calbiochem, San Diego, CA) and 1 mM phenylmethylsulphonyl fluoride] in a mortar, then adding more buffer to a ratio of 4:1 (vol:wt). Homogenates were cleared by filtering through Miracloth (Calbiochem) and spinning twice at 13,000 g for 15 min each to pellet cellular debris. Protein concentration in the resulting supernatant was then quantified by a modified Lowry assay, using BSA as a standard. Extracts were gently rocked with anti-GFP beads for one hour at room temperature. The beads were washed extensively (five times with ≥5× bead vol.) in extraction buffer, and the bound protein was eluted with low pH (0.1 M glycine pH 2.5) into a small amount of neutralizing 1 M tris buffer (pH 8.0), precipitated with TCA, separated by SDS-PAGE, blotted to PVDF membrane (Millipore, Billerica, MA), and probed with the appropriate antibodies. Positive signals were detected using HRP-conjugated secondary antibodies at 1:20,000 (Sigma).