Abstract
Plant survival requires the ability to acclimate to heat, which is involves the expression of heat-inducible genes. We found cytosolic heat shock protein (HSP) 90 serves as a negative regulator of heat shock transcription factor (HSF), which is responsible for the induction of heat-inducible genes in plant. Transient inhibition of HSP90 induces heat-inducible genes and heat acclimation in Arabidopsis thaliana seedlings. Most of upregulated genes by heat shock and HSP90 inhibitor treatments carry heat shock response element (HSE) in their promoter, which suggests that HSF participates in the response to HSP90 inhibition. A. thaliana HSP90.2 interacts with AtHsfA1d, which is one of the constitutively expressed HSFs in A. thaliana. Heat shock depleted cytosolic HSP90 activity, as shown by the activity of exogenously expressed glucocorticoid receptor (GR), which is a substrate of cytosolic HSP90. Thus, it appears that in the absence of heat shock, cytosolic HSP90 negatively regulates HsfA1. Upon heat shock, cytosolic HSP90 is transiently inactivated, and this may lead to the activation of HsfA1.
Key words: HSP90, heat shock transcription factor, heat shock, heat shock response element, heat acclimation, geldanamycin, radicicol
Although HSPs are responsible for refolding denatured proteins and/or for folding newly synthesized proteins, one of these HSPs, HSP90, also plays an additional role in the regulation of various cellular signaling molecules, including GR in animals and the pathogen recognition receptor, NBS-LRR-type R protein in plants. In A. thaliana, seven HSP90 homologues localize to the cytosol [HSP90.1 (At5g52640), HSP90.2 (At5g56030), HSP90.3 (At5g56010), and HSP90.4 (At5g56000)], mitochondria (At3g07770), chloroplast [CR88 (At2g04030)], and endoplasmic reticulum [SHD/GRP94 (At4g24190)]. The four cytosolic HSP90s are highly homologous to each other (86%–99% identity), which suggests that their activity is identical in A. thaliana. HSP90.2, HSP90.3 and HSP90.4 mRNAs accumulate constitutively, while HSP90.1 mRNA increases extensively after heat shock in A. thaliana seedlings.1 Hitherto, the function of cytosolic HSP90s has been mainly discussed within the context of the disease resistance in plant.2–6 However, it is likely that cytosolic HSP90s play other roles in intracellular events, since depletion of HSP90 activity induces a wide variety of morphological changes.7,8 Therefore, we investigated the effect of HSP90 inhibition to A. thaliana seedlings.
We used two chemicals, geldanamycin (GDA) and radicicol (RAD), to inhibit the function of HSP90. To investigate HSP90 functions on the gene regulation, we employed transgenic A. thaliana lines that bear β-glucuronidase (GUS) gene fused to various promoters. We found that a transgenic line that bears the heat-responsive HSP90.1 promoter connected to GUS (ProHSP90.1:GUS)9 gene induces the GUS after GDA or RAD treatment without heat shock. This indicates that GDA and RAD can induce heat-inducible genes in the absence of heat shock. This finding was further supported by a microarray analysis that revealed GDA or RAD treatment upregulated 157 heat-inducible genes in A. thaliana. Most of these genes are involved in heat shock protein/protein refolding (45 genes, 28.7%), transcription/translation (16 genes, 10.2%), and protein degradation (17 genes, 10.8%). Next, we searched the promoter regions of the upregulated 157 genes for the presence of a conserved motif. The promoters of 148 genes in the AGRIS database were analyzed by using the MEME program.10 The identified motif is three HSE repeat (HSE3) motif, which is a specific DNA sequence (nGAAnnT-TCnnGAAn) observed in the promoters of heat-inducible genes.11 Most of 148 genes (115 genes, 77.7%) had at least one HSE3-like motif in their promoter region, which suggests that HSE3 is responsible for the induction of these genes after heat shock or GDA or RAD treatments. In a minor population of 148 genes (15 genes. 10.1%), we also identified a conserved motif (CCACGGCT) that is closely related to the unfolded protein response (UPR) element of endoplasmic reticulum (ER) stress. We speculated that GDA and RAD inhibit SHD/GRP95, an ER-localized HSP90 homologue, thereby inducing UPR.
It is known that HSF is responsible for the induction of heat-inducible genes that have HSE3 in their promoter region in various organisms, including plants. Most HSFs form a trimer to bind to HSE3. Although animals and yeast have relatively few HSFs, plants have many HSF homologues that have been classified into A, B and C subfamilies.12 For example, A. thaliana has 15 class A, five class B and one class C HSFs, while tomato (Lycopersicon esculentum) has at least 11 class A, three class B and one class C HSFs.12,13 Plants use these HSFs in sophisticated heat shock response systems.12 For example, it has been shown that HsfA1 is constitutively expressed and a master regulator of heat shock response,14 HsfB1 is a coactivator of HsfA1,15 and HsfA2 and HsfA3 are induced by heat shock and enhance heat shock response.16–19 Several other HSFs seem to be involved in the regulation of thermal tolerance genes during development.12,20,21
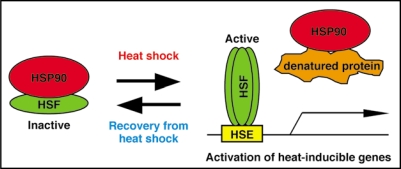
Like animals, plants constitutively have a pool of HSFs but do not induce heat-inducible genes without heat shock, which suggests that HSF activity is normally negatively regulated. In human cells, HSP90 interacts with HSF1 monomer and negatively regulates its function.22 Therefore, we hypothesized that cytosolic HSP90 in A. thaliana normally interacts with constitutively accumulated HSF to downregulate its function. Consistent with this, the effect of GDA and RAD on A. thaliana was not inhibited by co-treatment with cycloheximide, an inhibitor of translation, which suggests that the constitutively accumulated HSFs were mainly responsible for the induction of heat-inducible genes. We focused on AtHsfA1d because AtHsfA1d mRNA is expressed constitutively and AtHsfA1d protein recognizes HSE3. When we examined the interaction between A. thaliana HSP90.2 and AtHsfA1d by co-immunoprecipitation and bimolecular fluorescence complementation (BiFC) analyses, we found that HSP90.2 interacts with AtHsfA1d in the cytosol and nucleus. GDA or RAD treatment abolished the interaction between HSP90.2 and AtHsfA1d. These findings suggest that in the absence of heat shock, cytosolic HSP90 negatively regulates HsfA1 activity in plant (Fig. 1).
Figure 1.
A hypothetical model of HSP90 function in the heat shock response. HSP90 negatively regulates HSF in the absence of heat shock. After heat shock, HSP90 releas- es HSF and acts to refold denatured proteins. The free HSF forms a trimer and then binds to HSE3, thereby activating heat-inducible genes. HSP90 then downregulates heat-induced HSF during recovery from heat shock.
We then analyzed about the interactions between HSP90.2 and other A. thaliana HSFs. While AtHsfA4c mRNAs are expressed constitutively in A. thaliana seedlings, HSP90.2 did not bind to AtHsfA4c. This suggests that cytosolic HSP90 is not involved in the regulation of HsfA4. Recent findings show that HsfA5 is a negative regulator of HsfA4.23 The levels of AtHsfA7a and AtHsfB1 mRNAs are increased by heat shock, and HSP90.2 interacts with AtHsfA7a and AtHsfB1. This suggests cytosolic HSP90 also inactivates heat- inducible HSFs during the recovery from heat shock (Fig. 1).
It has been shown that prior heat shock leads to the acquisition of tolerance to high temperatures that are normally lethal.24,25 This so-called heat acclimation phenomenon enables plants to live without wilting in extremely hot environments. The above findings suggest that heat shock leads to the depletion of HSP90 activity, presumably because of the accumulation of denatured proteins, and that this induces HSF-mediated heat acclimation (Fig. 1). We measured HSP90 activity in vivo after heat shock by using a transgenic A. thaliana that exogenously expressed Gal4-VP16-GR (GVG).26 Since the GR domain of GVG is a substrate of HSP90, the GVG activity mirrors cytosolic HSP90 activity in the presence of dexamethasone, a ligand of GR. We found that HSP90 activity was depleted by heat shock, as well as GDA and RAD treatment. To address whether transient depletion of HSP90 activity is enough for heat acclimation, we transiently treated A. thaliana seedlings with GDA or RAD, and then subjected them to high temperatures. The HSP90 inhibitor-treated plants acquired high temperature tolerance without heat shock, which suggests that the transient inhibition of HSP90 is sufficient for heat acclimation in plants.
Since plants are sessile and cannot escape to cool places, they must respond promptly to heat stress. The HSP90-HSF complex may enable plants to rapidly sense heat damage and induce thermal tolerance genes. Two recent studies also suggest that HSP90 inhibition plays an important role in plant acclimation.3,27 Our findings complement these studies by elucidating the downstream events that occur after HSP90 inhibition. Interestingly, it has been suggested that the phosphorylation of HSF or the binding of HSP70 and small HSP to HSF may downregulate HSF activity.11,28 It is possible that HSP90 may regulate HSF activity in partnership with these molecules, thereby fine-tuning HSF activity in vivo.
Acknowledgements
This work was supported by a Grants-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan (no. 1977040 to K.Y. and no. 16085209 to M.N.).
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/5775
References
- 1.Yamada K, Fukao Y, Hayashi M, Fukazawa M, Suzuki I, Nishimura M. Cytosolic HSP90 regulates the heat shock response that is responsible for heat acclimation in Arabidopsis thaliana. J Biol Chem. 2007;282:37794–37804. doi: 10.1074/jbc.M707168200. [DOI] [PubMed] [Google Scholar]
- 2.Queitsch C, Sangster TA, Lindquist S. Hsp90 as a capacitor of phenotypic variation. Nature. 2002;417:618–624. doi: 10.1038/nature749. [DOI] [PubMed] [Google Scholar]
- 3.Sangster TA, Bahrami A, Wilczek A, Watanabe E, Schellenberg K, McLellan C, Kelley A, Kong SW, Queitsch C, Lindquist S. Phenotypic diversity and altered environmental plasticity in Arabidopsis thaliana with reduced Hsp90 levels. PLoS ONE. 2007;2:648. doi: 10.1371/journal.pone.0000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takabatake R, Ando Y, Seo S, Katou S, Tsuda S, Ohashi Y, Mitsuhara I. MAP kinases function downstream of HSP90 and upstream of mitochondria in TMV resistance gene N-mediated hypersensitive cell death. Plant Cell Physiol. 2007;48:498–510. doi: 10.1093/pcp/pcm021. [DOI] [PubMed] [Google Scholar]
- 5.Thao NP, Chen L, Nakashima A, Hara S, Umemura K, Takahashi A, Shirasu K, Kawasaki T, Shimamoto K. RAR1 and HSP90 form a complex with Rac/Rop GTPase and function in innate-immune responses in rice. Plant Cell. 2007;19:4035–4045. doi: 10.1105/tpc.107.055517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Botër M, Amigues B, Peart J, Breuer C, Kadota Y, Casais C, Moore G, Kleanthous C, Ochsenbein F, Shirasu K, Guerois R. Structural and functional analysis of SGT1 reveals that its interaction with HSP90 is required for the accumulation of Rx, an R protein involved in plant immunity. Plant Cell. 2007;19:3791–3804. doi: 10.1105/tpc.107.050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi A, Casais C, Ichimura K, Shirasu K. HSP90 interacts with RAR1 and SGT1 and is essential for RPS2-mediated disease resistance in Arabidopsis. Proc Natl Acad Sci USA. 2003;100:11777–11782. doi: 10.1073/pnas.2033934100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hubert DA, Tornero P, Belkhadir Y, Krishna P, Takahashi A, Shirasu K, Dangl JL. Cytosolic HSP90 associates with and modulates the Arabidopsis RPM1 disease resistance protein. EMBO J. 2003;22:5679–5689. doi: 10.1093/emboj/cdg547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yabe N, Takahashi T, Komeda Y. Analysis of tissue-specific expression of Arabidopsis thaliana HSP90-family gene HSP81. Plant Cell Physiol. 1994;35:1207–1219. doi: 10.1093/oxfordjournals.pcp.a078715. [DOI] [PubMed] [Google Scholar]
- 10.Molina C, Grotewold E. Genome wide analysis of Arabidopsis core promoters. BMC Genomics. 2005;6:25. doi: 10.1186/1471-2164-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schöffl F, Prandl R, Reindl A. Regulation of the heat-shock response. Plant Physiol. 1998;117:1135–1141. doi: 10.1104/pp.117.4.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Koskull-Döring D, Scharf KD, Nover L. The diversity of plant heat stress transcription factors. Trends Plant Sci. 2007;12:452–457. doi: 10.1016/j.tplants.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Nover L, Bharti K, Doring P, Mishra SK, Ganguli A, Scharf KD. Arabidopsis and the heat stress transcription factor world: how many heat stress transcription factors do we need? Cell Stress Chaperons. 2001;6:177–189. doi: 10.1379/1466-1268(2001)006<0177:aathst>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra SK, Tripp J, Winkelhaus S, Tschiersch B, Theres K, Nover L, Scharf KD. In the complex family of heat stress transcription factors, HsfA1 has a unique role as master regulator of thermotolerance in tomato. Gene Dev. 2002;16:1555–1567. doi: 10.1101/gad.228802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bharti K, von Koskull-Döring P, Bharti S, Kumar P, Tintschl Korbitzer A, Treuter E, Nover L. Tomato Heat stress transcription factor HsfB1 represents a novel type of general transcription coactivator with a histone-like motif interacting with with the plant CREB binding protein ortholog HAC1. Plant Cell. 2004;16:1521–1535. doi: 10.1105/tpc.019927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schramm F, Ganguli A, Kiehlmann E, Englich G, Walch D, von Koskull-Döring P. The heat stress transcription factor HsfA2 serves as a regulatory amplifer of a subset of genes in the heat stress response in Arabidopsis. Plant Mol Biol. 2006;60:759–772. doi: 10.1007/s11103-005-5750-x. [DOI] [PubMed] [Google Scholar]
- 17.Nishizawa A, Yabuta Y, Yoshida E, Maruta T, Yoshimura K, Shigeoka S. Arabidopsis heat shock transcription factor A2 as a key regulator in response to several types of environmental stress. Plant J. 2007;48:535–547. doi: 10.1111/j.1365-313X.2006.02889.x. [DOI] [PubMed] [Google Scholar]
- 18.Charng YH, Liu HC, Liu NY, Chi WT, Wang CN, Chang SH, Wang TT. A heat-inducible transcription factor, HsfA2, is required for expresion of aquired thermotolerance in Arabidopsis. Plant Physiol. 2007;143:251–262. doi: 10.1104/pp.106.091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schramm F, Larkindale J, Kiehlmann E, Ganguli A, Englich G, Vierling E, von Koskull-Döring P. A cascade of transcription factor DREB2A and heat stress transcription factor HsfA3 regulates the heat stress response of Arabidopsis. Plant J. 2008;53:264–274. doi: 10.1111/j.1365-313X.2007.03334.x. [DOI] [PubMed] [Google Scholar]
- 20.Kotak S, Vierling E, Bäumlein H, von Koskull-Döring P. A novel transcriptional cascade regulating expression of heat stress proteins during seed development of Arabidopsis. Plant Cell. 2007;19:182–195. doi: 10.1105/tpc.106.048165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamanouchi U, Yano M, Lin H, Ashikari M, Yamada K. A rice spotted leaf gene, Spl7, encodes a heat stress transcription factor protein. Proc Natl Acad Sci USA. 2002;99:7530–7535. doi: 10.1073/pnas.112209199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou J, Guo Y, Guettouche T, Smith DF, Voellmy R. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive comples with HSF1. Cell. 1998;94:471–480. doi: 10.1016/s0092-8674(00)81588-3. [DOI] [PubMed] [Google Scholar]
- 23.Baniwal SK, Chan KY, Scharf KD, Nover L. Role of heat stress transcription factor HsfA5 as specific repressor of HsfA4. J Biol Chem. 2007;282:3605–3613. doi: 10.1074/jbc.M609545200. [DOI] [PubMed] [Google Scholar]
- 24.Hong SW, Vierling E. Mutants of Arabidopsis thaliana defective in the acquisition of tolerance to high temperature stress. Proc Natl Acad Sci USA. 2000;97:4392–4397. doi: 10.1073/pnas.97.8.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Queitsch C, Hong SK, Vierling E, Lindquist S. Heat shock protein 101 plays a crucial role in thermotolerance in Arabidopsis. Plant Cell. 2000;12:479–492. doi: 10.1105/tpc.12.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aoyama T, Chua NH. A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J. 1997;11:605–612. doi: 10.1046/j.1365-313x.1997.11030605.x. [DOI] [PubMed] [Google Scholar]
- 27.McLellan CA, Turbyville TJ, Wijeratne EM, Kerschen A, Vierling E, Queitsch C, Whitesell L, Gunatilaka AA. A rhizosphere fungus enhances Arabidopsis thermotolerance through production of an HSP90 inhibitor. Plant Physiol. 2007;145:174–182. doi: 10.1104/pp.107.101808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prot M, Tripp J, Zielinski D, Weber C, Heerklotz D, Winkelhaus S, Bublak D, Scharf KD. Role of Hsp17.4-CII as coregulator and cytoplasmic regulation factor oftomato heat stress transcription factor HsfA2. Plant Physiol. 2004;135:1457–1470. doi: 10.1104/pp.104.042820. [DOI] [PMC free article] [PubMed] [Google Scholar]