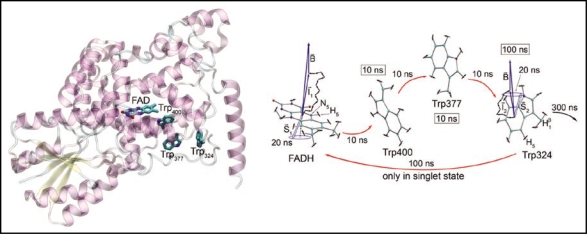
Figure 1.
Right: Cryptochrome internally binds the FAD cofactor and contains a three-tryptophan photoreduction pathway conserved from photolyase, consisting of Trp400, Trp377, and Trp324, with Trp400 nearest the FAD and Trp324 closest to the protein surface. After the FAD cofactor absorbs a photon, bringing it into an excited state, it is protonated from a nearby acidic residue, and then electron transfer proceeds from Trp400. At this stage, the semireduced FADH and Trp400+ comprise a radical pair—that is, each partner has an unpaired electron, and the spins of those electrons are in a correlated state. Cryptochrome is thought to be in its active, signaling state when the FAD cofactor is in this semireduced FADH form. An electron is then transferred from Trp377 to Trp400 and from Trp324 to Trp377, forming radical pairs FADH + Trp377+ and FADH + Trp324+ in the process. The Trp324 radical is then deprotonated. Before this final deprotonation, it is possible for the electron to back transfer from the tryptophan to FADH. If this occurs, FADH reverts to the oxidized FAD form, and cryptochrome is no longer in its active state. Left: This schematic of the electron transfer pathway in cryptochrome shows the estimated lifetimes of each of the radical pair states. The system spends most of its time in the FADH + Trp324 radical pair state. Also shown are the electron and nuclear spins on the FADH and Trp324 radicals. Each nuclear spin adds a small contribution to the local magnetic field. The unpaired electron spins are shown here in the singlet (antiparallel) state. They precess around the local magnetic field, which consists of contributions from the external field and from each of the nuclear spins, causing interconversion to the triplet (parallel) state and back again. This singlet-triplet interconversion is the basis of the radical pair effect in the following sense. Electron back-transfer from Trp324 to FADH proceeds only when the unpaired electrons on each radical are in the singlet state. Cryptochrome remains in its active state so long as this back-transfer is impeded. Therefore, singlet-triplet interconversion influences the time cryptochrome can spend in its active state, and so this magnetic-field-driven effect can alter the protein's signaling behavior.