Abstract
Ipomoea asarifolia and Turbina corymbosa (Convolvulaceae) are associated with epibiotic clavicipitalean fungi responsible for the presence of ergoline alkaloids in these plants. Experimentally generated plants devoid of these fungi were inoculated with different epibiotic and endophytic fungi resulting in a necrotic or commensal situation. A symbiotum of host plant and its respective fungus was best established by integration of the fungus into the morphological differentiation of the host plant. This led us to suppose that secretory glands on the leaf surface of the host plant may play an essential role in ergoline alkaloid biosynthesis which takes place in the epibiotic fungus.
Key words: ergoline alkaloids, ipomoea, turbina, convolvulaceae, claviceps, balansia, clavicipitaceae, penicillium, plant-fungus symbiotum
The Disjointed Occurrence of Ergoline Alkaloids in Nature is not Due to a Horizontal Gene Transfer
Ergoline (syn. ergot-) alkaloids are natural products of high physiological activity. They occur in clavicipitalean fungi colonising monocotyledonous plants belonging to the families Poaceae, Cyperaceae and Juncaceae. Ergoline alkaloids confer increased fitness, drought tolerance and feed deterrence to their host plants.1–3 The simultaneous occurrence of ergoline alkaloids in the dicotyledonous plant family Convolvulaceae was until recently an unexplained mystery which seemed to contradict the postulate of chemotaxonomy that similar or even identical natural products occur in related microbial or plant taxa.4 A horizontal gene transfer was often invoked to explain this phenomenon.1,3
This problem has now been solved by the observation that not only grasses but also Convolvulaceae plants [such as Ipomoea asarifolia Roem. et Schult, Turbina corymbosa (L.) Raf. (syn. Rivea corymbosa (L.) Hall.f.), and Ipomoea violacea L.] are colonized by seed transmitted epibiotic clavicipitalean fungi responsible for the biosynthesis and accumulation of ergoline alkaloids.
Evidence for this conclusion is given by the following observations:
The fungus is visible to the naked eye on the leaf surface or detectable by molecular biological techniques in seeds.5
The plant associated fungus contains the genetic material responsible for the biosynthesis of ergoline alkaloids.6
When surface sterilized seeds of I.asarifolia and T.corymbosa are grown in vitro under germ free condition the resulting plantlets are colonized exclusively by the respective clavicipitalean fungus (provisionally called IasaF13 or TcorF01). The plantlets contain ergoline alkaloids.7
Treatment of I.asarifolia and T.corymbosa with systemic azole fungicides eliminates both the epibiotic fungus and ergoline alkaloids.8
It was desirable to show that inoculation by epibiotic fungi (IasaF13 or TcorF01) of plants (I.asarifolia or T.corymbosa) deprived of all endophytes and epibionts restores ergoline alkaloid biosynthesis and accumulation in these plants. To this end we have carried out a series of inoculation experiments which characterizes the fungus/plant symbiotum. Techniques for the inoculation of host plants by their respective symbiotic fungi are available.9–11
Insights into the Fungus/Plant Association
In each of the experiments 1.1 to 5.2 (Table 1; A and B) a set of four plants were inoculated.7,9–11 Alternatively seeds and cell cultures were grown in the presence of an epibiotic fungus (Table 1; 6.1 and 6.2; C and D).7 Plants were repeatedly analysed for a possible fungal colonisation by microscopy between 10 to 26 weeks after the inoculation. The presence of ergoline alkaloids was checked by chromatographic techniques.5 The experiments were carried out in June 2004 and repeated in June 2005. Experimental details and results are summarized in Table 1.
Table 1.
Experiments designed to investigate the interaction between plants (I.asarifolia, T. corymbosa) and their clavicipitalean epibiotic fungi IasaF13 and TcorF01 and additional plant associated fungi
| Experiment no. | Host plant | Fungus | Inoculation technique | Result |
| 1.1 | I.asarifolia | IasaF13 | A | No fungal growth on inoculated plant visible; no alkaloids detectable within plant |
| 1.2 | T.corymbosa | TcorF01 | A | |
| 2.1 | I.asarifolia | IasaF13 | B | |
| 2.2 | T.corymbosa | TcorF01 | B | |
| 3.1 | I.asarifolia | B.obtecta | A | Necrosis; inoculated plants shed all leaves |
| 3.2 | T.corymbosa | B.obtecta | A | |
| 4.1 | I.asarifolia | C.purpurea | A | |
| 4.2 | T.corymbosa | C.purpurea | A | |
| 5.1 | I.asarifolia | Alternaria triticina Penicillium adametzioides Glomerella cingulata Sclerotinia sclerotiorum Penicillium olsonii | A | No incompatibility; fungi not established on plants; no ergoline alkaloids in host |
| 5.2 | I.asarifolia | P.roquefortii | A | Fungus well established on plant; no incompatibility; plant devoid of ergoline alkaloid (isofumigaclavin A) |
| 6.1 | I.asarifolia | IasaF13 | C | Fungus well established on plant; ergoline alkaloids present in plant |
| 6.2 | I.asarifolia | IasaF13 | D | Fungus well established on plant; ergoline alkaloids present in plant |
Plants devoid of epibiotic fungi and ergoline alkaloids (after fungicide treatment) were inoculated either by a suspension of fungal mycelium in water (A), or alternatively leaves covered with their epibiotic fungi (IasaF13 or TcorF01) were attached to leaves of plants deprived of their epibiotic fungi (“attachment experiment”) (B). Establishment of a symbiotum consisting of clavicipitalean fungus IasaF13 and I. asarifolia was achieved by regeneration of an intact I. asarifolia plant from a callus culture containing no alkaloids but the epibiotic fungus IasaF13 (C) and after growth of a surface sterilized seed under germ free condition(D). The interactions between fungi and host plants or plant cell culture are asymptomatic except in experiments 3.1 to 4.2. Experiments 1.1 to 2.2: Inoculation of host plants with their epibiotic ergoline alkaloid producing clavicipitalean fungi. Experiments 3.1 to 4.2: Inoculation of plants with grass borne endophytic clavicipitalean and ergoline alkaloid producing fungi. Experiments 5.1 and 5.2: Inoculation with endophytic from I.asarifolia isolated fungi including P.roquefortii, a producer of ergoline alkaloids e.g., isofumigaclavine A. Experiments 6.1 and 6.2: Integration of epibiotic clavicipitalean and ergoline alkaloid producing fungus IasaF13 into the developmental program of its host plant I. asarifolia.
Experimental inoculation of host plants with asexual epichloean endophytes is difficult and unlikely to occur in nature.12 This was also experienced in our inoculation experiments (Table 1; experiments 1.1 to 2.2) with both I. asarifolia and T. corymbosa and their respective epibiotic fungi IasaF13 and TcorF01. No colonisation of the phylloplane and no ergoline alkaloids within the leaves were detectable.
To investigate the infection process in more detail we used also symbiotic clavicipitalean fungi such as Balansia obtecta which is associated with the monocotyledonous plant Cenchrus echinatus (Poaceae). The fungus is known to produce ergoline alkaloids. Since this experiment resultet in a necrotic reaction of the inoculated dicotyledonous plants (Table 1) we also used Claviceps purpurea as an inoculant, a fungus which has a much broader host plant spectrum with more than 200 susceptible hosts.13 Again, a necrotic reaction in which the inoculated plants shed all leaves occurred (Table 1). These observations are again in line with experiences which had been encountered with grass plants and their symbiotic fungi:“… each mutualistic grass-endophyte symbiosis appears to require close coadaptation of host and endophyte.”12 The beneficial relation between plant associated fungi and their host requires a fine tuned interaction.
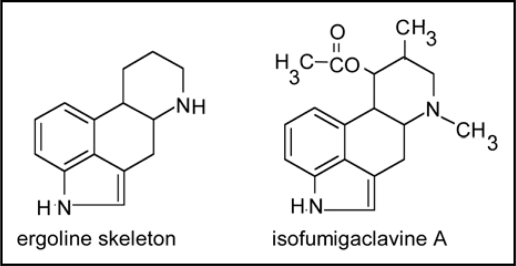
As expected, an asymptomatic association was experienced when I.asarifolia was inoculated with five different endophytes which had been isolated from this plant and employed in various combinations for the inoculation experiment (Table 1; experiment 5.1). Accumulation of ergoline alkaloids was not observed in these cases indicating that these fungi may not be responsible for ergoline alkaloid biosynthesis. This, however, was not necessarily expected in the next experiment (Table 1, experiment 5.2) in which I.asarifolia was inoculated with Penicillium roquefortii. This fungus was isolated from I.asarifolia under experimental conditions yielding different endophytes. Penicillium roquefortii is a producer of ergo-line alkaloids notably isofumigaclavine A (Fig. 1).14 The fungus was asymptomatically well established on the plant within 3 weeks without preferred contact to glandular cells. Alkaloids, however, were not detectable within the inoculated plant.
Figure 1.
Isofumigaclavine A, an ergoline alkaloid produced by Penicillium roqufortii.
Final proof for the role of the clavicipitalean fungus IasaF13 was obtained, when the fungus was integrated into the developmental program of its host plant I.asarifolia: Plant callus and cell suspension cultures of this plant were devoid of ergoline alkaloids. These cultures, however, contained the fungus IasaF13,7 which apparently survived the sterilisation procedure during establishment of the cell culture. The fungus was detectable by single strand conformation polymorphism (SSCP), sequencing of its internal transcribed spacer DNA and by microscopy.7 Both plant cells and fungus survive in the culture without any hypersensitive response apparently keeping each other in check. Change of the hormone regime of the culture led to a regenerated plant covered with its epibiotic clavicipitalean fungus IasaF13 and ergoline alkaloid accumulation within the plant (Table 1, experiment 6.1).
The same result was achieved when a surface sterilized seed was germinated under germ free conditions. The resulting plantlet was colonized by the fungus IasaF13 demonstrating that this fungus transmitted with the seed, spreads within and over the plant during growth and must be responsible for the ergoline alkaloid accumulation within the plant (Table 1, experiment 6.2).6 Both experiments 6.1 and 6.2 show that morphological differentiation of the host plant is essential for the biosynthesis of ergoline alkaloids produced by the fungus which is hitherto not permanently cultivable in vitro.
We have previously observed that the epibiotic fungi IasaF13 and TcorF01 are closely associated with secretory glands on the adaxial leaf surface,8 an anatomical feature which may be essential for ergoline alkaloid biosynthesis in the epibiotic fungus/plant association. This assumption would explain why cell cultures are devoid of ergoline alkaloids: They harbor the fungus but develop no secretory glands.
The possible role of secretory glands in ergoline alkaloid biosynthesis may also be highlighted by the observation that P. roquefortii is well established on the host plant I.asarifolia and is capable to synthesize ergoline alkaloids. Microscopic inspection of the leaf surface shows, however, that the fungus is not associated with secretory glands. We speculate that this could be the reason why the plant is devoid of isofumigaclavine A.
The secretory glands and their volatile oil may be mediators of a metabolic dialogue between both associated organisms. It is conceivable that:
the volatile oil is involved in signalling during the interaction between fungus and plant.
the fungus feeds on the volatile oil and derives precursors for ergoline alkaloid biosynthesis from the oil.
At present we cannot exclude that both assumptions are correct.
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft to E.L.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/6432
References
- 1.Clay K, Schardl C. Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. Am Nat. 2002;160:99–127. doi: 10.1086/342161. [DOI] [PubMed] [Google Scholar]
- 2.Schardl C, Pannacione DG, Tudzynsky P. Ergot-alkaloids-biology and molecular biology. In: Cordell GA, editor. The Alkaloids: Chemistry and Biology. Vol. 63. New York: Academic Press; 2006. pp. 45–86. [DOI] [PubMed] [Google Scholar]
- 3.Groeger D, Floss HG. Biochemistry of ergot alkaloids-achievements and challenges. In: Cordell GA, editor. The alkaloids: Chemistry and Biology. Vol. 50. New York: Academic Press; 1998. pp. 171–218. [Google Scholar]
- 4.Hofmann A. Die Entdeckung einer Wunderdroge. München: Deutscher Taschenbuchverlag; 2006. LSD-mein Sorgenkind. (Ger). [Google Scholar]
- 5.Ahimsa-Müller MA, Markert A, Hellwig S, Knoop V, Steiner U, Drewke C, Leistner E. Clavicipitaceous fungi associated with ergoline alkaloid-containing Convolvulaceae. J Nat Prod. 2007;70:1955–1960. doi: 10.1021/np070315t. [DOI] [PubMed] [Google Scholar]
- 6.Markert A, Steffan N, Ploss K, Hellwig S, Steiner U, Drewke C, Li SM, Boland W, Leistner E. Biosynthesis and accumulation of ergoline alkaloids in a mutualistic association between Ipomoea asarifolia (Convolvulaceae) and a clavicipitalean fungus. Plant Physiol. 2008;147:296–305. doi: 10.1104/pp.108.116699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steiner U, Ahimsa-Müller MA, Markert A, Kucht S, Groß J, Kauf N, Kuzma M, Zych M, Lamshöft M, Furmanowa M, Knoop V, Drewke C, Leistner E. Molecular characterisation of a seed transmitted clavicipitaceous fungus occurring on dicotyledoneous plants (Convolvulaceae) Planta. 2006;224:533–544. doi: 10.1007/s00425-006-0241-0. [DOI] [PubMed] [Google Scholar]
- 8.Kucht S, Groß J, Hussein Y, Grothe T, Keller U, Basar S, König WA, Steiner U, Leistner E. Elimination of ergoline alkaloids following treatment of Ipomoea asarifolia (Convolvulaceae) with fungicides. Planta. 2004;219:619–625. doi: 10.1007/s00425-004-1261-2. [DOI] [PubMed] [Google Scholar]
- 9.Latch GCM, Christensen MJ. Artificial infection of grasses with endophytes. Ann Appl Biol. 1985;107:17–24. [Google Scholar]
- 10.Schardl CL, Leuchtmann A. The Epichloë endophytes of grasses and the symbiotic continuum. In: Dighton J, White JF Jr, Oudemans P, editors. Mycology, Vol 23. The Fungal Community, its Organisation and Role in the Ecosystems. Boca Raton: CRC Taylor and Francis; 2005. pp. 475–503. [Google Scholar]
- 11.Pannacione DG, Johnson RD, Wang J, Young CA, Damrongkool P, Scott B, Schardl CL. Elimination of ergovaline from a grass-Neotyphodium endophyte symbiosis by genetic modification of the endophyte. Proc Natl Acad Sci USA. 2001;98:820–825. doi: 10.1073/pnas.221198698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gentile A, Rossi MS, Cabral D, Craven KD, Schardl CL. Origin, divergence and phylogeny of epichloe endophytes of native Argentine grasses. Mol Phylogenet Evol. 2005;35:196–208. doi: 10.1016/j.ympev.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Alderman SC. Diversity and Speciation in Claviceps. In: White JF Jr, Bacon CW, Hywel-Jones, Spatafora JW, editors. Clavicipitalean Fungi, Evolutionary Biology, Chemistry, Biocontrol and Cultural Impacts. Vol. 19. New York and Basel: Marcel Dekker; 2003. pp. 195–245. [Google Scholar]
- 14.Scott PM, Merrien MA, Polonsky J. Roquefortine and isofumigaclavine A, metabolites from Penicillium roquefortii. Experientia. 1976;32:140–142. [Google Scholar]