Abstract
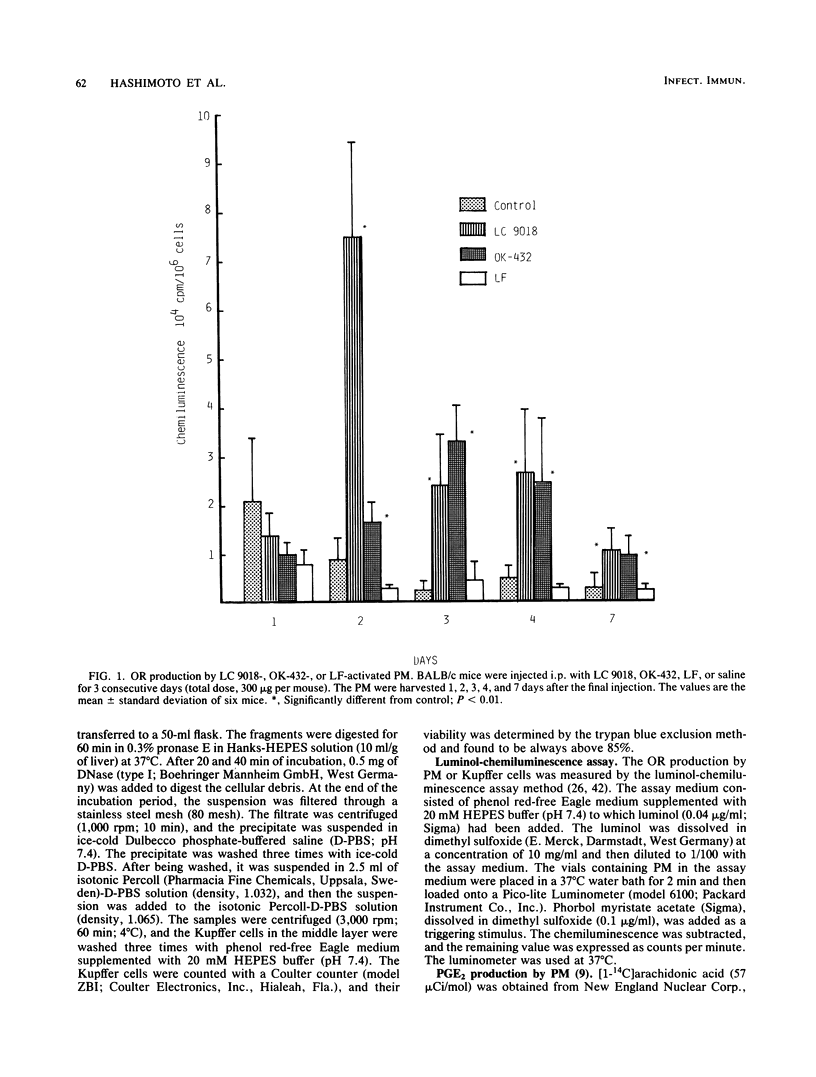
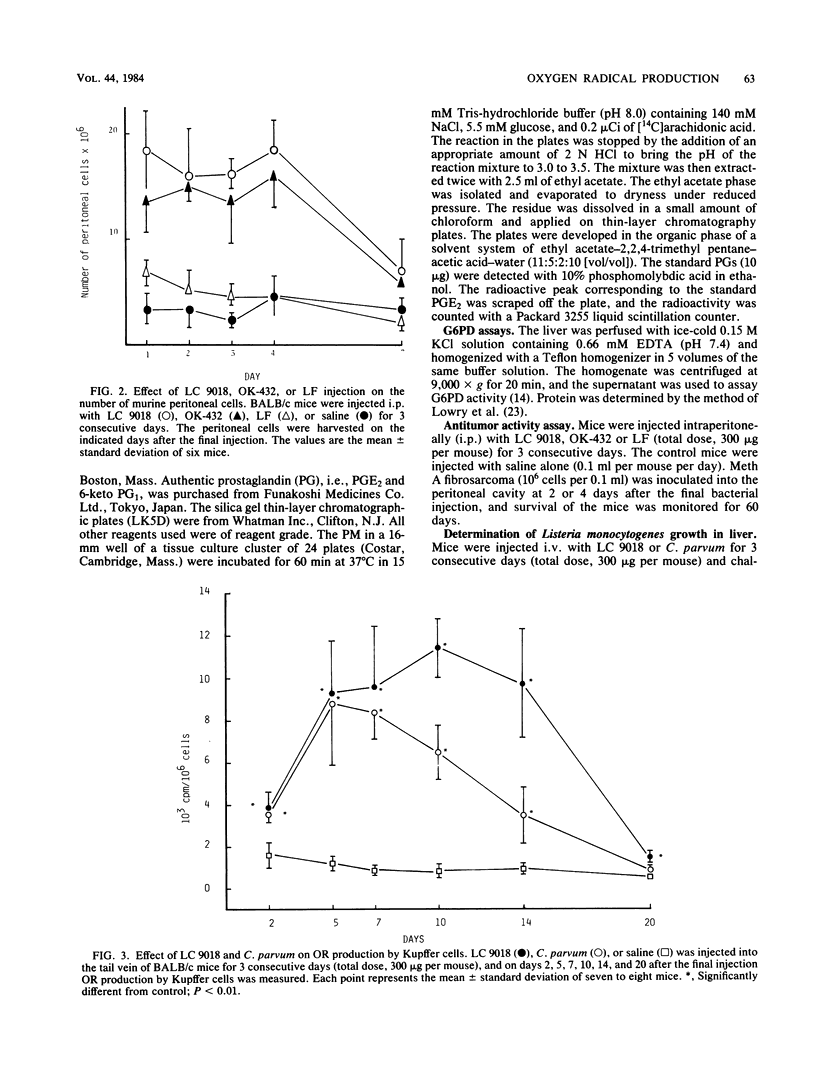
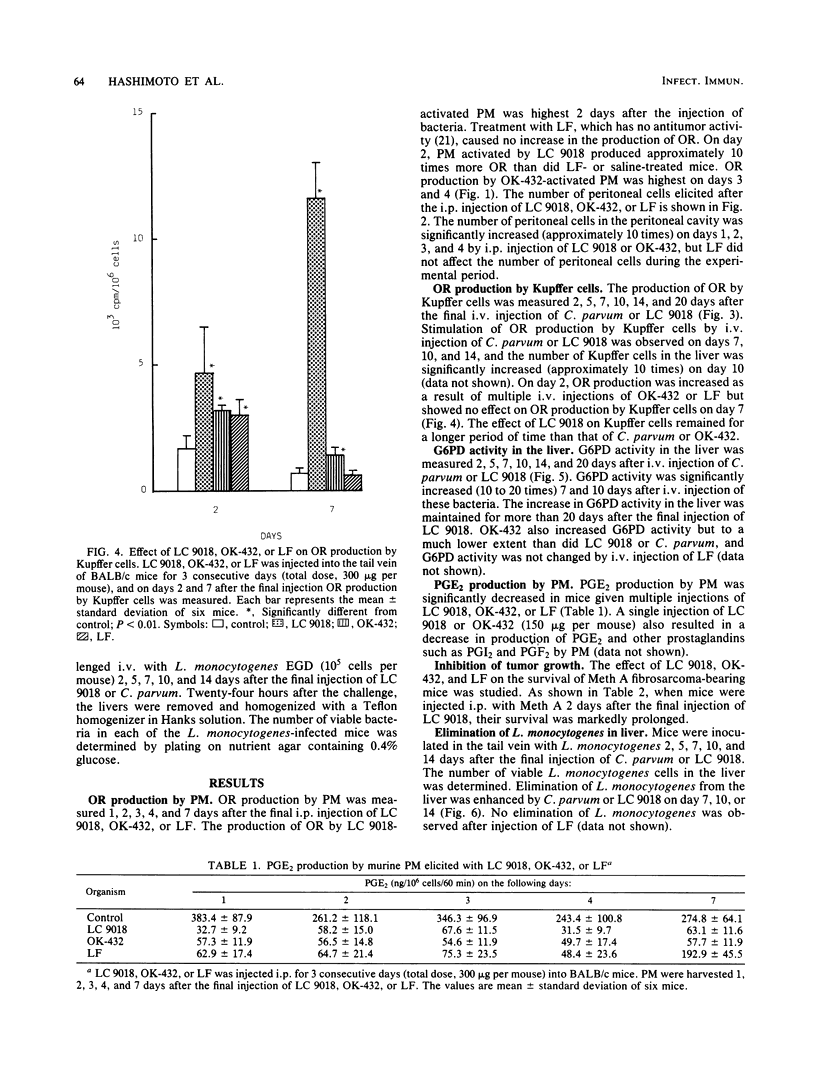
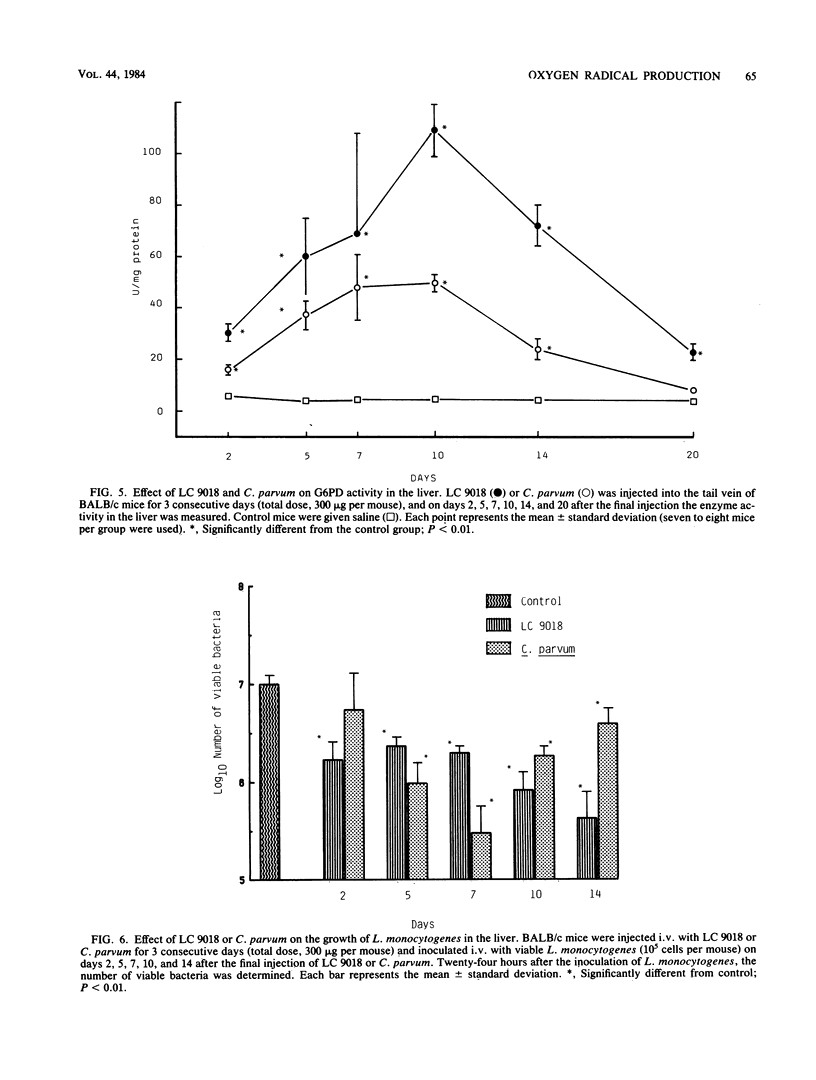
BALB/c mice were injected intraperitoneally (i.p.) or intravenously (i.v.) with Lactobacillus casei YIT9018 (LC 9018). The i.p. injected LC 9018 augmented oxygen radical (OR) production by peritoneal macrophages (PM) and suppressed the production of prostaglandin E2 by PM. The growth of i.p. inoculated Meth A fibrosarcoma was also inhibited by an i.p. injection of LC 9018. i.v. injection of LC 9018 stimulated OR production by fixed macrophages and inhibited the growth of Listeria monocytogenes in the liver. Furthermore, glucose-6-phosphate dehydrogenase activity in the liver was significantly increased (10 to 20 times) by LC 9018 i.v. injection. A significant correlation was observed between the augmentation of OR production by PM or fixed macrophages in the liver and inhibition of growth of Meth A or L. monocytogenes. The augmentation of OR production by LC 9018 was more marked and was maintained for a longer period of time than that by other bacterial immunostimulants.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Johnson W. J., Fiorito E., Nathan C. F. Hydrogen peroxide and cytolytic factor can interact synergistically in effecting cytolysis of neoplastic targets. J Immunol. 1981 Nov;127(5):1973–1977. [PubMed] [Google Scholar]
- Adams D. O., Kao K. J., Farb R., Pizzo S. V. Effector mechanisms of cytolytically activated macrophages. II. Secretion of a cytolytic factor by activated macrophages and its relationship to secreted neutral proteases. J Immunol. 1980 Jan;124(1):293–300. [PubMed] [Google Scholar]
- Auclair C., Torrès M., Hakim J. Involvement of hydroxyl radical in NAD(P)H oxidation and associated oxygen reduction by the granule fraction of human blood polymorphonuclears. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1067–1072. doi: 10.1016/0006-291x(78)91244-5. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Bonney R. J., Naruns P., Davies P., Humes J. L. Antigen-antibody complexes stimulate the synthesis and release of prostaglandins by mouse peritoneal macrophages. Prostaglandins. 1979 Oct;18(4):605–616. doi: 10.1016/0090-6980(79)90027-3. [DOI] [PubMed] [Google Scholar]
- Chang W. C., Nakao J., Neichi T., Orimo H., Murota S. Effects of estradiol on the metabolism of arachidonic acid by aortas and platelets in rats. Biochim Biophys Acta. 1981 May 22;664(2):291–297. [PubMed] [Google Scholar]
- Cohen S. A., Salazar D., Nolan J. P. Natural cytotoxicity of isolated rat liver cells. J Immunol. 1982 Aug;129(2):495–501. [PubMed] [Google Scholar]
- Crofton R. W., Diesselhoff-den Dulk M. M., van Furth R. The origin, kinetics, and characteristics of the Kupffer cells in the normal steady state. J Exp Med. 1978 Jul 1;148(1):1–17. doi: 10.1084/jem.148.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie G. A. Activated macrophages kill tumour cells by releasing arginase. Nature. 1978 Jun 29;273(5665):758–759. doi: 10.1038/273758a0. [DOI] [PubMed] [Google Scholar]
- Diesselhoff-den Dulk M. M., Crofton R. W., van Furth R. Origin and kinetics of Kupffer cells during an acute inflammatory response. Immunology. 1979 May;37(1):7–14. [PMC free article] [PubMed] [Google Scholar]
- GLOCK G. E., McLEAN P. Further studies on the properties and assay of glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase of rat liver. Biochem J. 1953 Oct;55(3):400–408. doi: 10.1042/bj0550400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S., Dobrjansky A. Corynebacterium parvum: effects on the biochemistry of mouse serum and liver. J Natl Cancer Inst. 1979 Aug;63(2):497–502. [PubMed] [Google Scholar]
- Humes J. L., Burger S., Galavage M., Kuehl F. A., Jr, Wightman P. D., Dahlgren M. E., Davies P., Bonney R. J. The diminished production of arachidonic acid oxygenation products by elicited mouse peritoneal macrophages: possible mechanisms. J Immunol. 1980 May;124(5):2110–2116. [PubMed] [Google Scholar]
- Ishii Y., Yamaoka H., Toh K., Kikuchi K. Inhibition of tumor growth in vivo and in vitro by macrophages from rats treated with a streptococcal preparation, OK-432. Gan. 1976 Feb;67(1):115–119. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaku M., Yagawa K., Nagao S., Tanaka A. Enhanced superoxide anion release from phagocytes by muramyl dipeptide or lipopolysaccharide. Infect Immun. 1983 Feb;39(2):559–564. doi: 10.1128/iai.39.2.559-564.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato I., Kobayashi S., Yokokura T., Mutai M. Antitumor activity of Lactobacillus casei in mice. Gan. 1981 Aug;72(4):517–523. [PubMed] [Google Scholar]
- Kato I., Yokokura T., Mutai M. Macrophage activation by Lactobacillus casei in mice. Microbiol Immunol. 1983;27(7):611–618. doi: 10.1111/j.1348-0421.1983.tb00622.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matthews N. Production of an anti-tumour cytotoxin by human monocytes: comparison of endotoxin, interferons and other agents as inducers. Br J Cancer. 1982 Apr;45(4):615–617. doi: 10.1038/bjc.1982.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M. Free radicals and inflammation: protection of synovial fluid by superoxide dismutase. Science. 1974 Aug 9;185(4150):529–531. doi: 10.1126/science.185.4150.529. [DOI] [PubMed] [Google Scholar]
- Metzger Z., Hoffeld J. T., Oppenheim J. J. Regulation by PGE2 of the production of oxygen intermediates by LPS-activated macrophages. J Immunol. 1981 Sep;127(3):1109–1113. [PubMed] [Google Scholar]
- Miake S., Takeya K., Matsumoto T., Yoshikai Y., Nomoto K. Relation between bactericidal and phagocytic activities of peritoneal macrophages induced by irritants. J Reticuloendothel Soc. 1980 Apr;27(4):421–427. [PubMed] [Google Scholar]
- Mitsuyama M., Takeya K., Nomoto K., Shimotori S. Three phases of phagocyte contribution to resistance against Listeria monocytogenes. J Gen Microbiol. 1978 May;106(1):165–171. doi: 10.1099/00221287-106-1-165. [DOI] [PubMed] [Google Scholar]
- Munthe-Kaas A. C., Berg T., Seglen P. O., Seljelid R. Mass isolation and culture of rat kupffer cells. J Exp Med. 1975 Jan 1;141(1):1–10. doi: 10.1084/jem.141.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F. Secretion of oxygen intermediates: role in effector functions of activated macrophages. Fed Proc. 1982 Apr;41(6):2206–2211. [PubMed] [Google Scholar]
- Nathan C., Nogueira N., Juangbhanich C., Ellis J., Cohn Z. Activation of macrophages in vivo and in vitro. Correlation between hydrogen peroxide release and killing of Trypanosoma cruzi. J Exp Med. 1979 May 1;149(5):1056–1068. doi: 10.1084/jem.149.5.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan J. P. Endotoxin, reticuloendothelial function, and liver injury. Hepatology. 1981 Sep-Oct;1(5):458–465. doi: 10.1002/hep.1840010516. [DOI] [PubMed] [Google Scholar]
- Parant M. A., Parant F. J., Chedid L. A. Enhancement of resistance to infections by endotoxin-induced serum factor from Mycobacterium bovis BCG-infected mice. Infect Immun. 1980 Jun;28(3):654–659. doi: 10.1128/iai.28.3.654-659.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertoft H., Rubin K., Kjellén L., Laurent T. C., Klingeborn B. The viability of cells grown or centrifuged in a new density gradient medium, Percoll(TM). Exp Cell Res. 1977 Dec;110(2):449–457. doi: 10.1016/0014-4827(77)90311-1. [DOI] [PubMed] [Google Scholar]
- Roos E., Dingemans K. P., Van de Pavert I. V., Van den Bergh-Weerman M. A. Mammary-carcinoma cells in mouse liver: infiltration of liver tissue and interaction with Kupffer cells. Br J Cancer. 1978 Jul;38(1):88–99. doi: 10.1038/bjc.1978.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruco L. P., Meltzer M. S. Macrophage activation for tumor cytotoxicity: development of macrophage cytotoxic activity requires completion of a sequence of short-lived intermediary reactions. J Immunol. 1978 Nov;121(5):2035–2042. [PubMed] [Google Scholar]
- Saito H., Tomioka H. Enhanced hydrogen peroxide release from macrophages stimulated with streptococcal preparation OK-432. Infect Immun. 1979 Nov;26(2):779–782. doi: 10.1128/iai.26.2.779-782.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffet S. M., Pace J. L., Russell S. W. Lymphokine maintains macrophage activation for tumor cell killing by interfering with the negative regulatory effect of prostaglandin E2. J Immunol. 1981 Jul;127(1):121–124. [PubMed] [Google Scholar]
- Taffet S. M., Russell S. W. Macrophage-mediated tumor cell killing: regulation of expression of cytolytic activity by prostaglandin E. J Immunol. 1981 Feb;126(2):424–427. [PubMed] [Google Scholar]
- Toh K., Yamamoto N., Kikuchi K. Modulation of cell growth by isolated Kupffer cells. J Reticuloendothel Soc. 1981 Apr;29(4):265–274. [PubMed] [Google Scholar]
- Viadana E., Bross I. D., Pickren J. W. An autopsy study of some routes of dissemination of cancer of the breast. Br J Cancer. 1973 Apr;27(4):336–340. doi: 10.1038/bjc.1973.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse E., Knook D. L. The investigation of sinusoidal cells: a new approach to the study of liver function. Prog Liver Dis. 1979;6:153–171. [PubMed] [Google Scholar]


