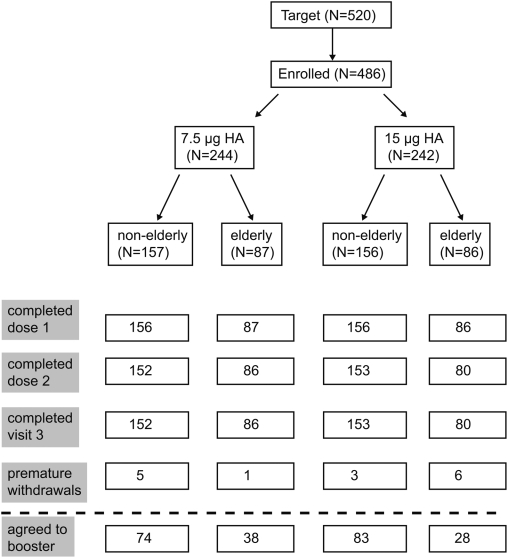
Figure 1. Subject disposition.
A total of 486 healthy adults were enrolled in the study and randomized in a 1∶1 ratio, stratified by age group (non-elderly adults, aged 18–60 years, and elderly adults, aged >60 years) to receive two vaccinations of either 7.5 μg or 15 μg HA H5N1 (A/Vietnam/1194/2004; NIBRG-14) inactivated subunit influenza virus vaccine adjuvanted with MF59. The first two vaccinations were administered 21 days apart, and a subset of the first participants also received a third (booster) vaccination 6 months later.