Figure 6.
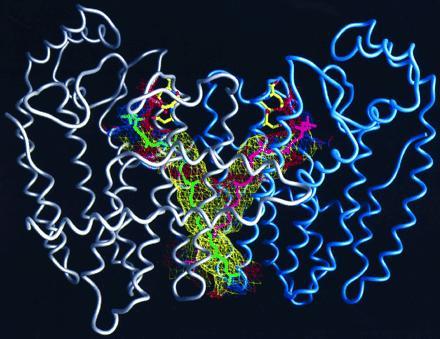
Structure of mutant F112A/F113S-FPS dimer with two C-40 isoprenes, shown in green and magenta, modeled into proposed binding channels. The first subunit of the dimer is shown with a gray α-carbon worm, and the second is in blue. The diphosphate heads of the two isoprenes are shown binding to the aspartates of the first conserved DDXXD sequences of each subunit in the same configuration observed for DMAPP, GPP, and FPP. The walls of the channels that contain the growing hydrophobic tails of the prenyl products are shown in mesh. The mesh is colored according to the hydrophobicity of the protein atoms that it is composed of, with yellow being hydrophobic, red acidic, and blue basic.
